Matter ANY THING THAT HAS MASS AND TAKES











- Slides: 15

Matter ANY THING THAT HAS MASS AND TAKES UP SPACE

Matter is composed of many small particles that are called atoms. l Atoms are microscopic, and help to give the matter its fixed shape, or very flexible shape. l

States of Matter Solid Liquid Gas

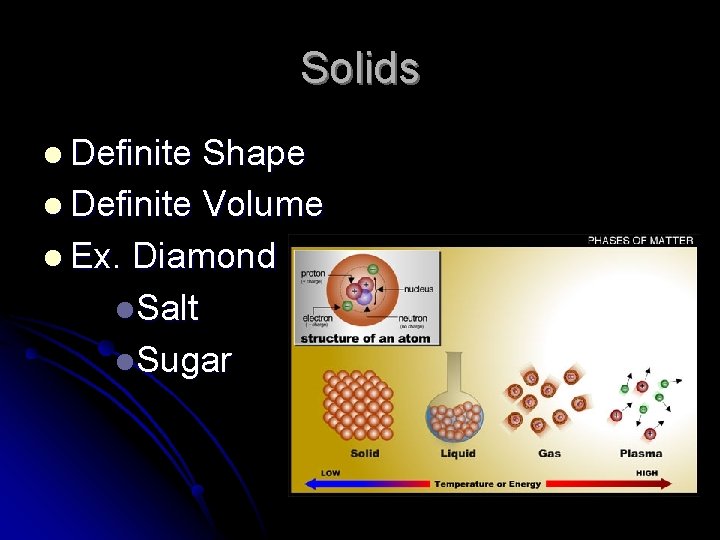
Solids l Definite Shape l Definite Volume l Ex. Diamond l. Salt l. Sugar

Types of Solids Crystalline l A solid that is made up of crystals in which particles are arranged in a regular, repeating pattern l Ex. Salt l. Sugar l. Snow l Melts at a specific Temperature

Types of Solids l Amorphous l. A solid made up of particles that are not arranged in a regular pattern l Ex. Plastics l. Rubber l. Glass l. Does not melt at a distinct temperature

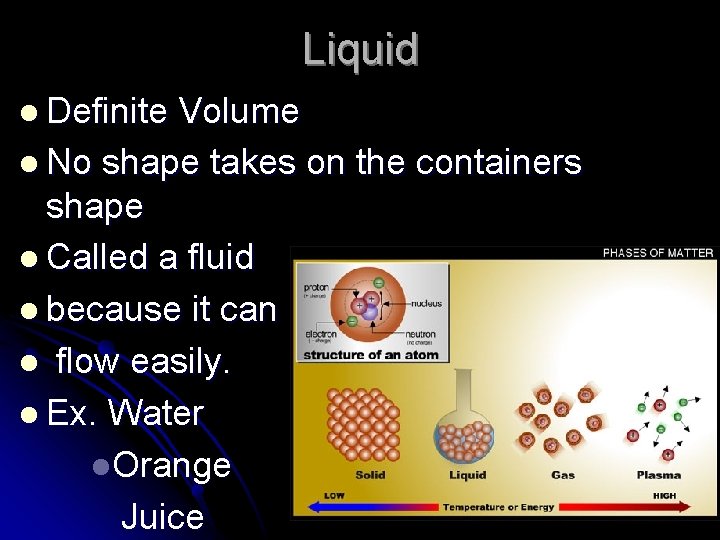
Liquid l Definite Volume l No shape takes on the containers shape l Called a fluid l because it can l flow easily. l Ex. Water l. Orange Juice

Properties of Liquids l Surface Tension l. The tightness across the surface of water that caused by polar molecules pulling on one another l Viscosity l. A liquids resistance to flow

l No Gases definite Shape l No Definite Volume l The particles are free to move freely in the space they are given l Ex Oxygen l. Carbon Dioxide

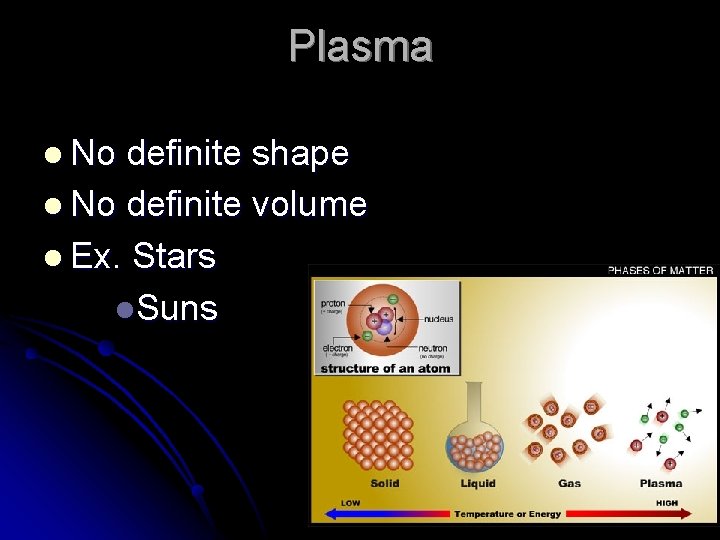
Plasma l No definite shape l No definite volume l Ex. Stars l. Suns

Changes of State l Melting l Solid to a liquid l Ice- At 0°C ice stops increasing, and then starts to melt into water. l Melting Point l The specific temperature at which a substance changes from a solid to a liquid

Changes of State l Freezing l Liquid to a solid l Particles stop moving to begin to form a regular pattern. l Freezing Point l The specific temperature at which a substance changes from a liquid to a solid l Freezing point and melting point for water is the same

Changes of State l Evaporation l Liquid to Gas l. Vaporization that takes place only on the surface of a liquid

Changes of State l Boiling l. Occurs when a liquid changes to a gas below the surface l Boiling Point l. Specific temperature at which a substance boils

Changes in State Condensation l Gas to a liquid l Occurs when particles in a gas lose enough thermal energy to form a liquid. l Sublimation l Solid to a gas l Occurs when the surface particles of a solid gain enough energy that they form a gas. They do not pass through the liquid state. l Example- dry ice l
 Anything that has mass and occupies space is called?
Anything that has mass and occupies space is called? Anything that takes up space and has mass is
Anything that takes up space and has mass is Matter anything that takes up space
Matter anything that takes up space Matter is anything that
Matter is anything that Anything defintion
Anything defintion Matter is anything
Matter is anything Anything that has mass
Anything that has mass Matter is anything that has
Matter is anything that has Matter is anything that has
Matter is anything that has What is anything that has mass and volume called
What is anything that has mass and volume called Anything that takes up space and has mass
Anything that takes up space and has mass Matter is anything that has mass and volume.
Matter is anything that has mass and volume. It is anything that has mass and occupies space
It is anything that has mass and occupies space Matter is anything that has and occupies
Matter is anything that has and occupies Phân độ lown ngoại tâm thu
Phân độ lown ngoại tâm thu Block xoang nhĩ ecg
Block xoang nhĩ ecg


























