Matter ANY THING THAT HAS MASS AND TAKES











- Slides: 15

Matter ANY THING THAT HAS MASS AND TAKES UP SPACE

Matter is composed of many small particles that are called atoms. l Atoms are microscopic, and help to give the matter its fixed shape, or very flexible shape. l

States of Matter Solid Liquid Gas

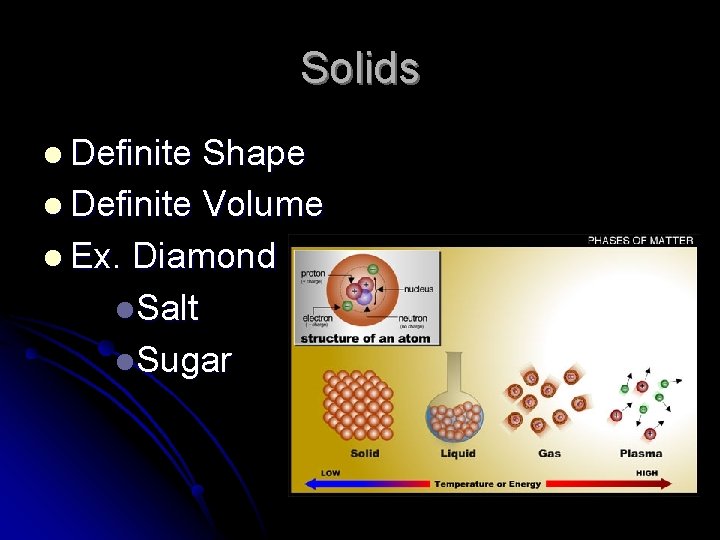
Solids l Definite Shape l Definite Volume l Ex. Diamond l. Salt l. Sugar

Types of Solids Crystalline l A solid that is made up of crystals in which particles are arranged in a regular, repeating pattern l Ex. Salt l. Sugar l. Snow l Melts at a specific Temperature

Types of Solids l Amorphous l. A solid made up of particles that are not arranged in a regular pattern l Ex. Plastics l. Rubber l. Glass l. Does not melt at a distinct temperature

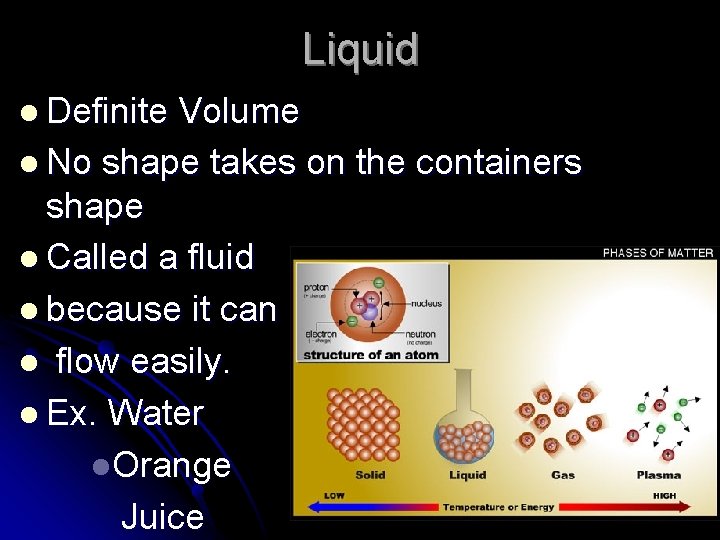
Liquid l Definite Volume l No shape takes on the containers shape l Called a fluid l because it can l flow easily. l Ex. Water l. Orange Juice

Properties of Liquids l Surface Tension l. The tightness across the surface of water that caused by polar molecules pulling on one another l Viscosity l. A liquids resistance to flow

l No Gases definite Shape l No Definite Volume l The particles are free to move freely in the space they are given l Ex Oxygen l. Carbon Dioxide

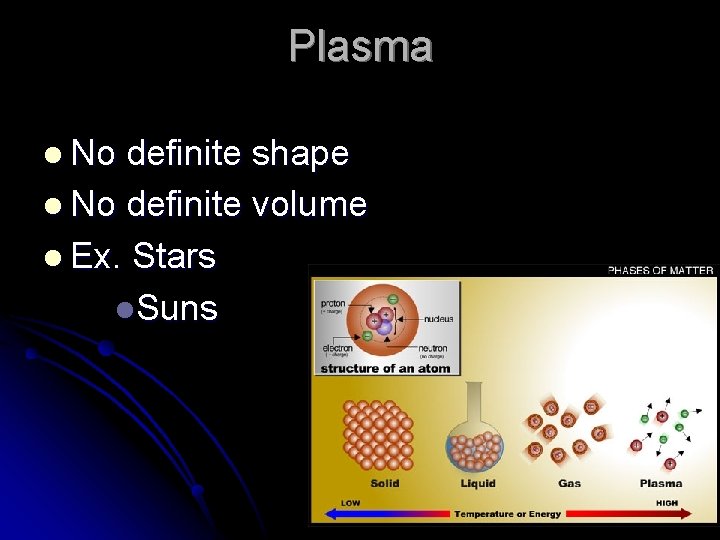
Plasma l No definite shape l No definite volume l Ex. Stars l. Suns

Changes of State l Melting l Solid to a liquid l Ice- At 0°C ice stops increasing, and then starts to melt into water. l Melting Point l The specific temperature at which a substance changes from a solid to a liquid

Changes of State l Freezing l Liquid to a solid l Particles stop moving to begin to form a regular pattern. l Freezing Point l The specific temperature at which a substance changes from a liquid to a solid l Freezing point and melting point for water is the same

Changes of State l Evaporation l Liquid to Gas l. Vaporization that takes place only on the surface of a liquid

Changes of State l Boiling l. Occurs when a liquid changes to a gas below the surface l Boiling Point l. Specific temperature at which a substance boils

Changes in State Condensation l Gas to a liquid l Occurs when particles in a gas lose enough thermal energy to form a liquid. l Sublimation l Solid to a gas l Occurs when the surface particles of a solid gain enough energy that they form a gas. They do not pass through the liquid state. l Example- dry ice l