Matter and the Study of Chemistry Matter Anything





























- Slides: 33

Matter and the Study of Chemistry

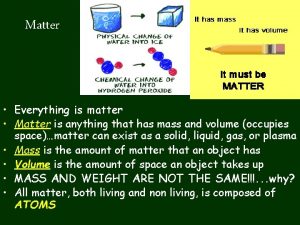
Matter: Anything that has mass and takes up space All matter is composed of chemicals Characteristics: Mass vs. weight Mass is amount of matter Weight is the force of gravity on an object Inertia – resistance to change in motion Energy – ability to do work (potential and kinetic)

The Law of Conservation of Energy The Law of Conservation of Matter

Example: water Composition: hydrogen and oxygen Structure: Properties: Physical – liquid at room temperature, boils at 100 C Chemical – splits into hydrogen and oxygen when subjected to electric current 2 H 2 O 2 H 2 + O 2

Physical and. Chemical Properties & Physical and Chemical Changes

Physical Properties: those properties that define a substance -density -color -texture state or phase boiling point solubility

Physical changes are those changes that do not result in the production of a new substance. If you melt a block of ice, you still have H 2 O at the end of the change.

If you break a bottle, you still have glass. Painting your nails will not stop them from being fingernails. Some common examples of physical changes are: melting, freezing, condensing, breaking, crushing, cutting, and bending.

Some, but not all physical changes can be reversed. You could refreeze the water into ice, but you cannot put your hair back together if you don’t like your haircut!

Special types of physical changes where any object changes state, such as when water freezes or evaporates, are sometimes called change of state operations.

CHEMICAL PROPERTIES Chemical properties can ONLY be observed AS the substances are changing into different substances.

Chemical changes, or chemical reactions, are changes that result in the production of another substance.

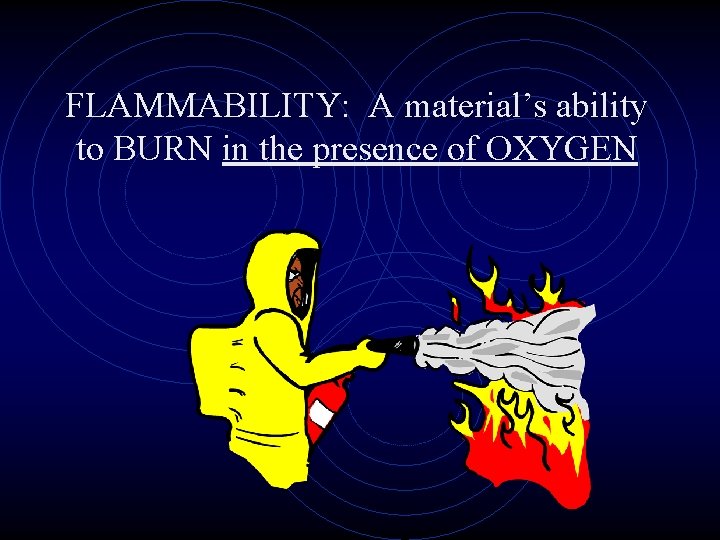
FLAMMABILITY: A material’s ability to BURN in the presence of OXYGEN

REACTIVITY: How readily (easily) a substance combines chemically with other substances.

Which has higher reactivity? A 14 karat gold ring or a cheap metal ring from the vending machine at the grocery store? What is your evidence?

When you burn a log in a fireplace, you are carrying out a chemical reaction that releases carbon. When you light your Bunsen burner in lab, you are carrying out a chemical reaction that produces water and carbon dioxide.

Common examples of chemical changes that you may be somewhat familiar with are; digestion, respiration, photosynthesis, burning, and decomposition.

Physical or Chemical Change? • Painting Wood • PHYSICAL

Physical or Chemical Change? • Burning Paper • CHEMICAL

Physical or Chemical Change? • Digestion of food • CHEMICAL

Physical or Chemical Change? • Sugar dissolving in water • PHYSICAL

Physical or Chemical Change? • Iron turning red when heated • PHYSICAL

Physical or Chemical Change? • Evaporation • PHYSICAL

Physical or Chemical Change? • A pond freezing in winter • PHYSICAL

Physical or Chemical Change? • Melting ice • PHYSICAL

Physical or Chemical Change? • Cutting wire • PHYSICAL

Physical or Chemical Change? • Painting fingernails • PHYSICAL

Physical or Chemical Change? • Cutting fabric • PHYSICAL

Physical or Chemical Change? • Baking muffins • CHEMICAL

Physical or Chemical Change? • Shattering glass • PHYSICAL

Physical or Chemical Change? • Decomposition of old leaves • CHEMICAL

Physical or Chemical Change? • Wrinkling a shirt • PHYSICAL

Physical or Chemical Change? • An old nail rusting • CHEMICAL
 Anything that takes up space and has mass is
Anything that takes up space and has mass is Anything that occupies space and has mass is called
Anything that occupies space and has mass is called Matter is anything that has mass and volume
Matter is anything that has mass and volume Matter anything that takes up space
Matter anything that takes up space Matter is anything that has and occupies
Matter is anything that has and occupies Matter is anything that has mass and
Matter is anything that has mass and All matter has and takes up
All matter has and takes up Matter is anything that occupies space
Matter is anything that occupies space Boiling point defintion
Boiling point defintion Anything that has mass
Anything that has mass Matter is defined as anything that
Matter is defined as anything that No matter anything
No matter anything Matter anything that
Matter anything that Matter is anything that...
Matter is anything that... Defintion of matter
Defintion of matter Ionized matter
Ionized matter Matter is anything that:
Matter is anything that: No matter anything
No matter anything Matter is anything that
Matter is anything that Matter anything that
Matter anything that Matter anything that
Matter anything that Benjamin cummings
Benjamin cummings Matter anything that
Matter anything that Matter anything that
Matter anything that Definition of substance
Definition of substance Chemistry matter and change chapter 7
Chemistry matter and change chapter 7 Chemistry: matter and change chapter 10 the mole answer key
Chemistry: matter and change chapter 10 the mole answer key Chemistry matter and change chapter 7
Chemistry matter and change chapter 7 Chemistry matter and change chapter 6
Chemistry matter and change chapter 6 Chapter 10 study guide the mole
Chapter 10 study guide the mole Chemistry matter and change chapter 2 answer key
Chemistry matter and change chapter 2 answer key Ib organic chemistry
Ib organic chemistry Inorganic vs organic chemistry
Inorganic vs organic chemistry Gray matter in the brain
Gray matter in the brain