Matter and its properties Matter defined Matter is













- Slides: 16

Matter and its properties

• Matter defined: – Matter is anything that has mass and takes up space • Volume defined: – The amount of space an object takes up • Units expressed as m. L, or cm cubed

Mass and Weight, What’s the difference • Mass is the amount of matter in an object. – Unit expressed by grams – Always constant, never changes • Weight is a measure of the gravitational force exerted on an object. – Unit expressed in Newtons (N) – Can change, depending on the objects location in the universe.

Physical Properties of Matter • Let’s play 20 questions!

Identifying Matter • Thermal Conductivity – The rate at which a substance transfers heat.

Identifying Matter • Density – The mass per unit volume of a substance, or m/v • Lead is a very dense metal • Solubility – The ability of a substance to dissolve in another substance

Identifying Matter • Ductility – The ability of a substance to be pulled into a wire. • Malleability – The ability of a substance to be rolled or pounded into thin sheets

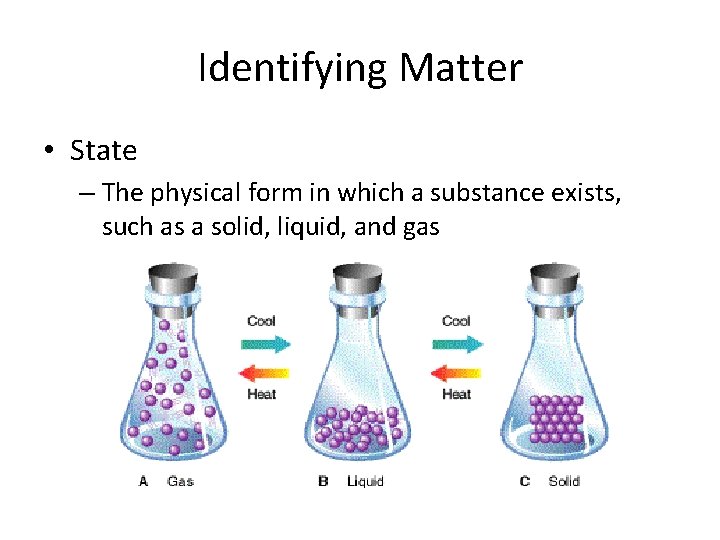
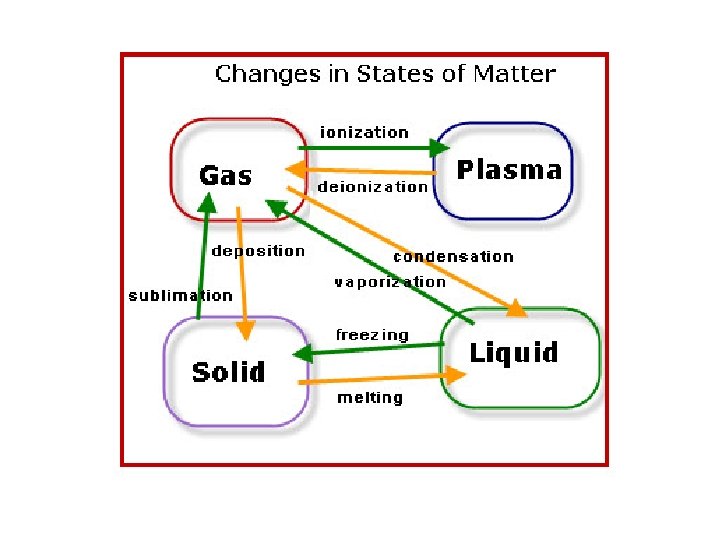
Identifying Matter • State – The physical form in which a substance exists, such as a solid, liquid, and gas

New State! • Let me introduce you to the fourth state of matter: – PLASMA

Physical Changes • A physical change is a change that affects one or more physical properties of a substance, but does not change the identity of the matter involved. Many Physical changes can be reversed.

Chemical Properties • Chemical properties describe matter based on its ability to change into new matter that has different properties. – Examples: Wood has the chemical property of flammability, because it can burn. – Ash and smoke can not burn, so they have the chemical property of nonflammability.

• More Examples of Chemical Properties – Reactivity: the ability of two or more substances to combine and form one or more new substances

Physical and Chemical Properties

Chemical Changes • A chemical change happens when one or more substances are changed into new substances that have new and different properties. -The statue of liberty was originally orange, because it is made of copper. Copper is reactive with oxygen, it turns green.

How do you know if a Chemical Change has occurred? • Indication a chemical change has occurred: – Change in color – Odor – Production of heat – Fizzing or foaming – Sound or light • Almost all chemical changes are irreversible, like baking a cake. Electrolysis is an example of one that can be reversed – The method of sending an electric current into water, so it can split and form hydrogen and oxygen
