Matter and How It Changes Ch 13 What





















- Slides: 29

Matter and How It Changes Ch. 13

What is matter? List as many types of matter as you can in the space below.

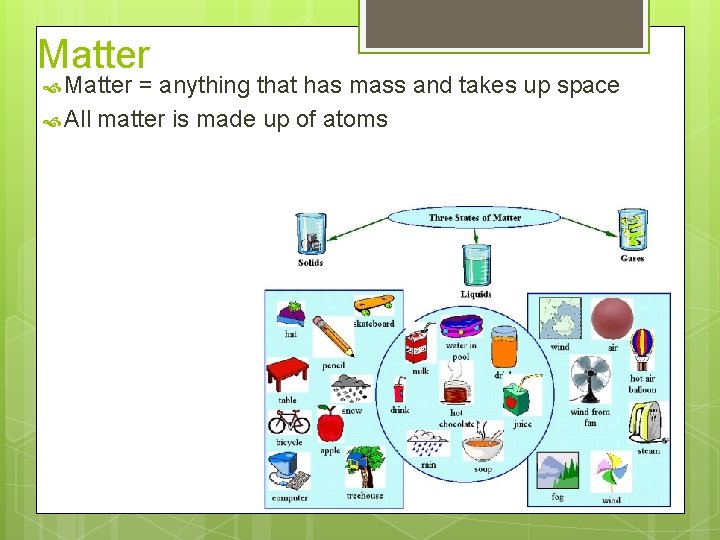
Matter = anything that has mass and takes up space All matter is made up of atoms

Physical Properties of Matter Physical Property = something that describes a substance by itself and can be observed or measured without changing the identity of the substance.

Physical Property: Mass = the amount of matter something has Can be measured in grams (g) or kilograms (kg) Does not depend on gravity like weight does

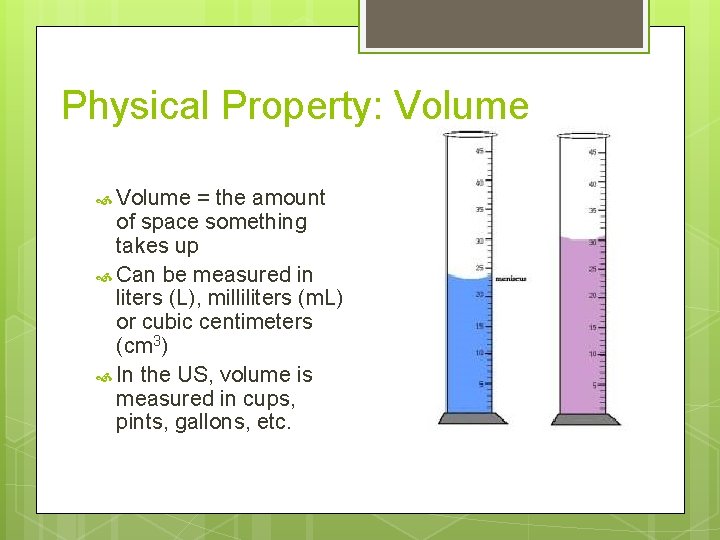
Physical Property: Volume = the amount of space something takes up Can be measured in liters (L), milliliters (m. L) or cubic centimeters (cm 3) In the US, volume is measured in cups, pints, gallons, etc.

Physical Property: Density = the amount of mass something has in relation to its volume Can be measured in Grams per liter (g/L) Grams per cubic centimeter (g/cm 3) Grams per milliliter (g/m. L)

Density A substance will float in another substance if it is less dense than that substance A substance will sink in another substance if it is more dense than that substance

Physical Changes Physical change = when one or more physical properties of a substance undergoes a change, but it does not become a different substance

Physical Properties Dissolving: Solubility = a measure of how much a substance will dissolve in a liquid Melting Boiling Evaporating = turning from a liquid to a gas

Mixtures Mixture =a combination of 2 or more substances that keep their original properties

Separating Mixtures Can be easy or difficult to do. Can you think of a mixture that would be easy to separate? Can you think of a mixture that would be difficult to separate?

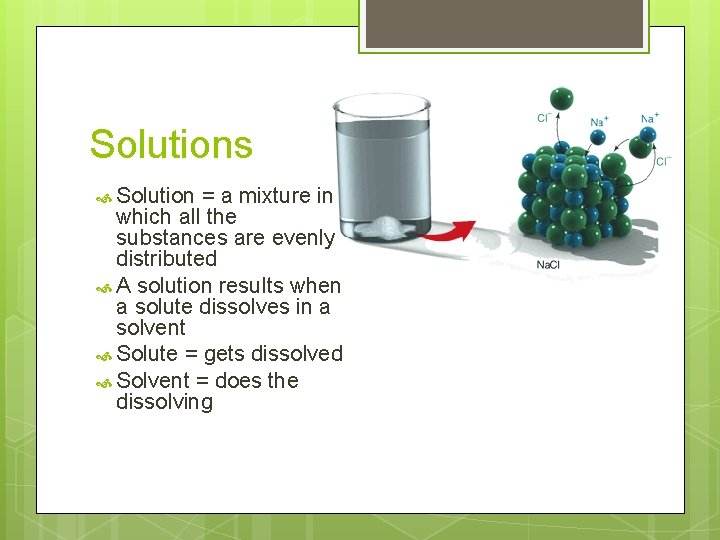
Solutions Solution = a mixture in which all the substances are evenly distributed A solution results when a solute dissolves in a solvent Solute = gets dissolved Solvent = does the dissolving

Solubility = a measure of how much a substance will dissolve in a given solvent Is salt soluble in water? Yes or no Is sand soluble in water? Yes or no

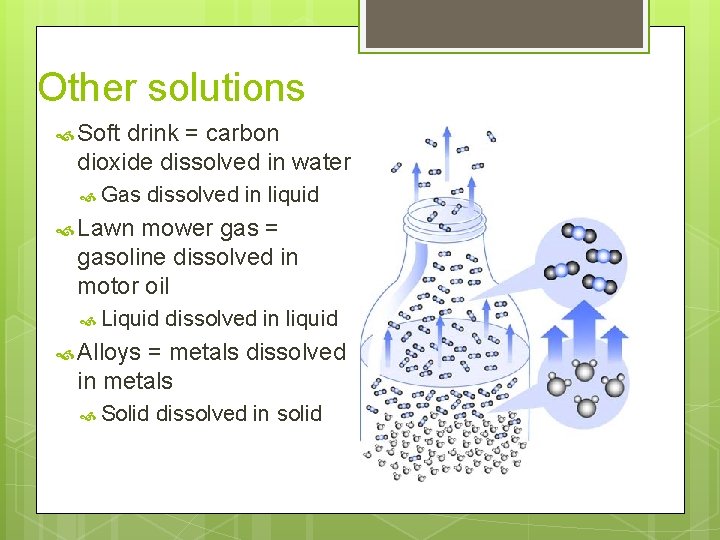
Other solutions Soft drink = carbon dioxide dissolved in water Gas dissolved in liquid Lawn mower gas = gasoline dissolved in motor oil Liquid dissolved in liquid Alloys = metals dissolved in metals Solid dissolved in solid

Alloy = mixture of metals Brass = copper + zinc Bronze = copper + tin Pewter = tin + antimony + copper Steel = iron + manganese + carbon Stainless steel = steel + chromium + nickel

Other Mixtures Suspension = a uniform mixture that contains particles that are large enough to be seen mixing soil in water At first, you will see bits of soil floating around in the water After an hour, the soil will all be settled at the bottom Suspensions mixed don’t stay

Other Mixtures Colloid = a mixture that contains particles that are too small to see A colloid is just like a suspension but because the particles are so small they don’t settle to the bottom Milk is an example of a colloid – as long as it is refrigerated, it won’t separate

Other Mixtures Emulsion = a mixture of two liquids that don’t dissolve Example = mayonnaise Mayo is an emulsion of Vegetable oil Egg yolks Vinegar The vegetable oil and vinegar don’t mix

Chemical Change Chemical change = a change in which one or more new substances are formed. To find out if a chemical change has occurred, look at the substances chemical properties. What is a chemical property?

Chemical Property Chemical property = something that describes the ability of a substance to react with other materials and form new substances Releases heat Produces gas Changes color Produces a smell

Reactivity and Stability Reactivity = the ability of a substance to go through a chemical change Stability = the ability of a substance to resist going through a chemical change

Preventing chemical changes Removing Putting heat: food in the refrigerator to stop it from spoiling Packaging food in cans Developing photographs in a dark room

Acids Acid = a substance that turns blue litmus paper red Examples: Lemon juice Orange juice Vinegar Car battery acid Lime juice

Bases Base = a substance that turns red litmus paper blue Examples: Ammonia Laundry detergent Draino Baking Soap soda

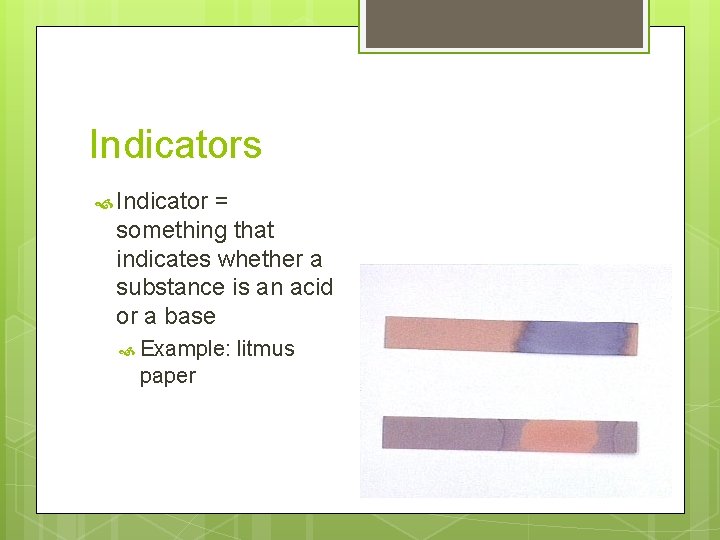
Indicators Indicator = something that indicates whether a substance is an acid or a base Example: paper litmus

Strengths of acids and bases Weak acids Lemon juice Vinegar Strong acids Battery acid Stomach acid Weak bases Soap Baking Strong bases Draino Lye soda

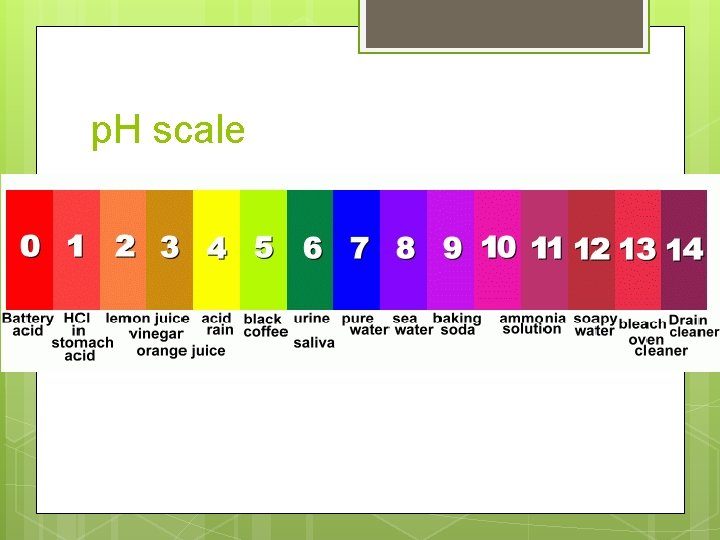
p. H scale = a measure of the strength or weakness of acids or bases Ranges from 0 – 14 0 – 6 = acid 8 – 14 = base 7 = neutral 0 = strongest acid 14 = strongest base

p. H scale