Matter And Energy The Nature of Matter Gold









- Slides: 28

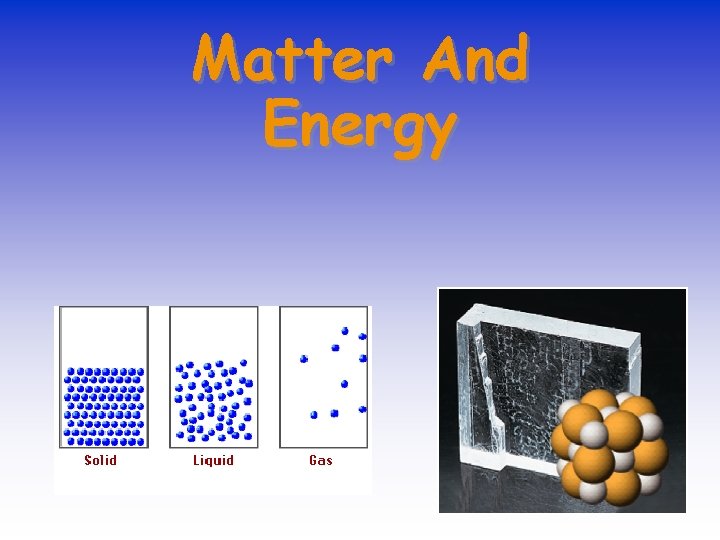
Matter And Energy

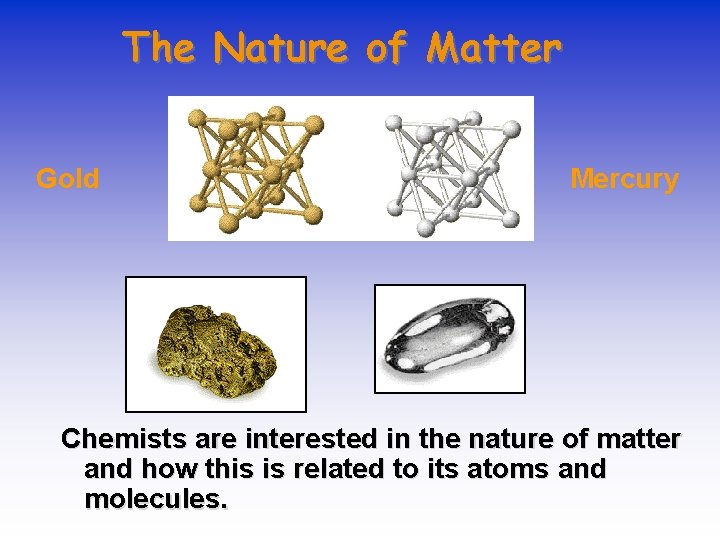
The Nature of Matter Gold Mercury Chemists are interested in the nature of matter and how this is related to its atoms and molecules.

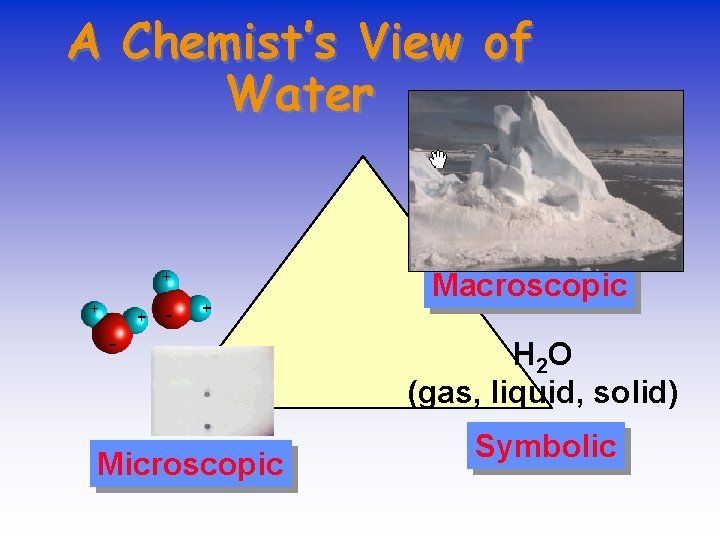
Chemistry & Matter • We can explore the MACROSCOPIC world — what we can see — • to understand the MICROSCOPIC worlds we cannot see. • We write SYMBOLS to describe these worlds.

A Chemist’s View of Water Macroscopic H 2 O (gas, liquid, solid) Microscopic Symbolic

Kinetic Theory of Matter 1. Molecules are always moving. This is known as the kinetic theory of matter 2. We measure this kinetic energy with a thermometer as temperature 3. The greater the material’s internal energy, the higher the temperature of that material. 4. Heat is energy flow between objects of different temperatures 5. Heat and temperature are NOT the same 6. Brownian motion describes how visible particles are seen moving due to invisible molecules bumping into them

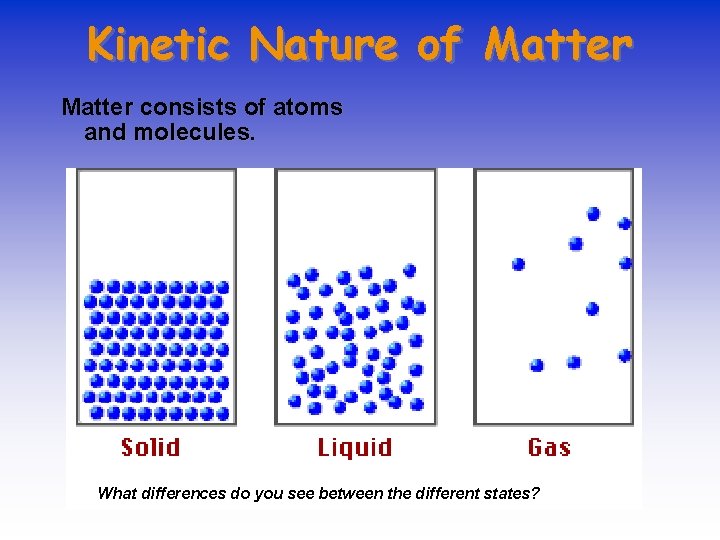
Kinetic Nature of Matter consists of atoms and molecules. What differences do you see between the different states?

STATES OF MATTER • _______ — have rigid shape, fixed volume. External shape can reflect the atomic and molecular arrangement. – Little space between atoms, little energy – Most dense state (one exception) • _______ — have no fixed shape and may not fill a container completely. – Space between molecules, medium energy – Have a medium density • _______ — expand to fill their container. – A lot of space between molecules, High energy – Have very low density

OTHER STATES OF MATTER • PLASMA — an electrically charged gas; Example: the sun or any other star

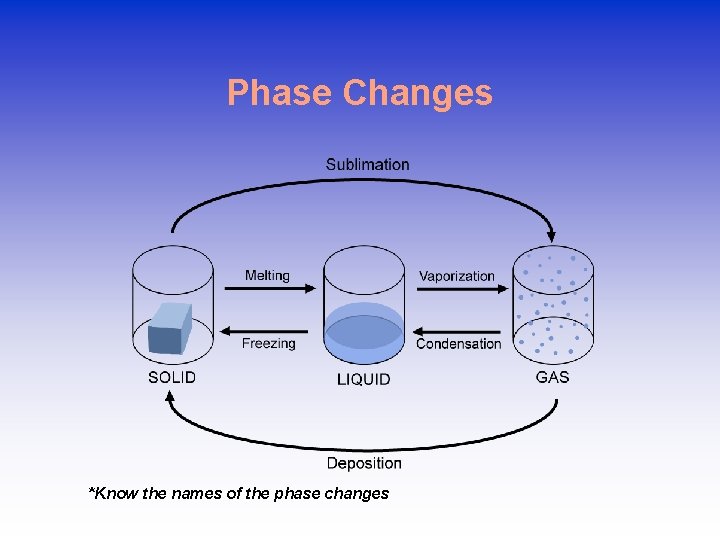
Phase Changes *Know the names of the phase changes

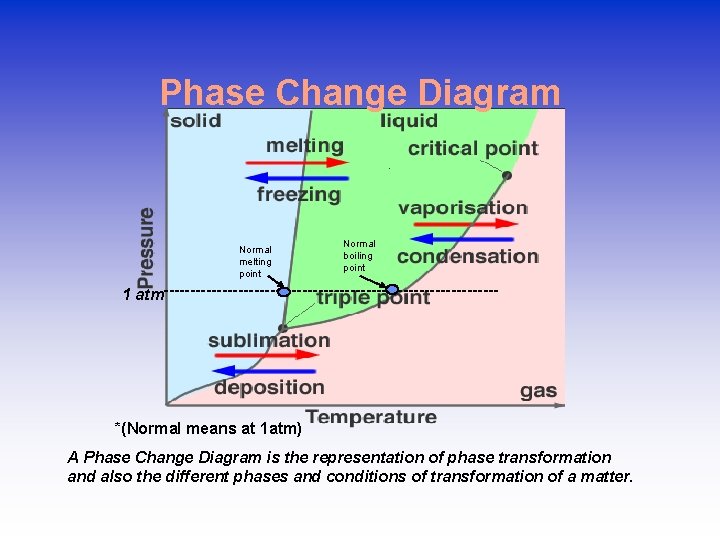
Phase Change Diagram Normal melting point Normal boiling point 1 atm-------------------------------- *(Normal means at 1 atm) A Phase Change Diagram is the representation of phase transformation and also the different phases and conditions of transformation of a matter.

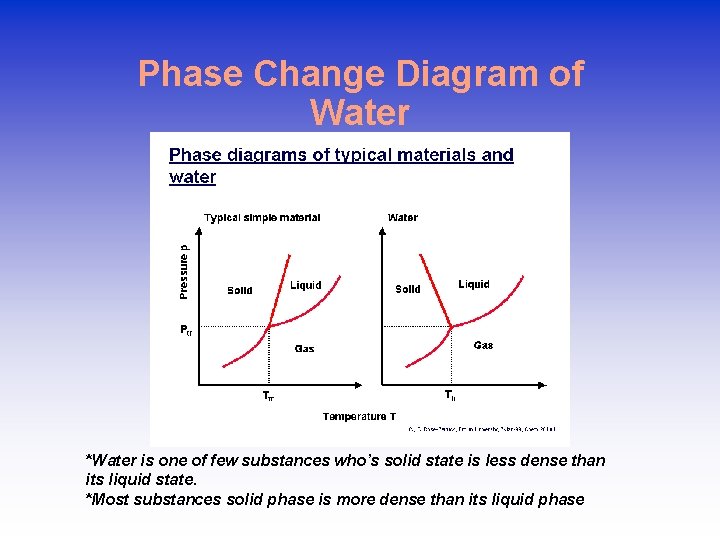
Phase Change Diagram of Water *Water is one of few substances who’s solid state is less dense than its liquid state. *Most substances solid phase is more dense than its liquid phase

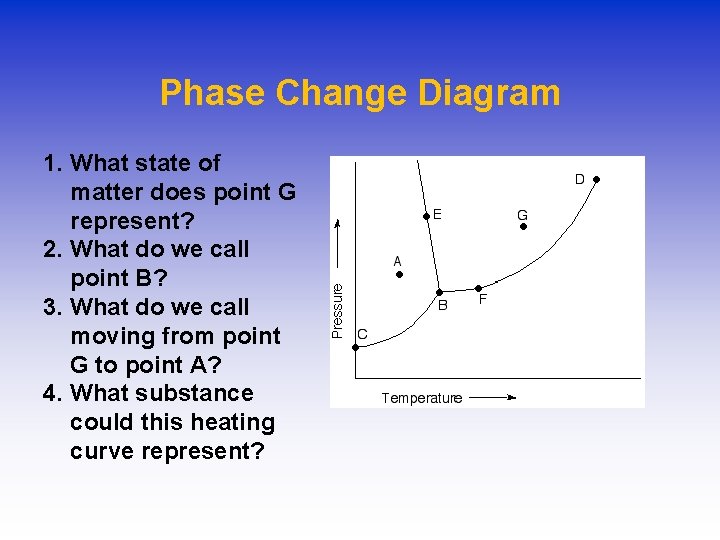
Phase Change Diagram 1. What state of matter does point G represent? 2. What do we call point B? 3. What do we call moving from point G to point A? 4. What substance could this heating curve represent?

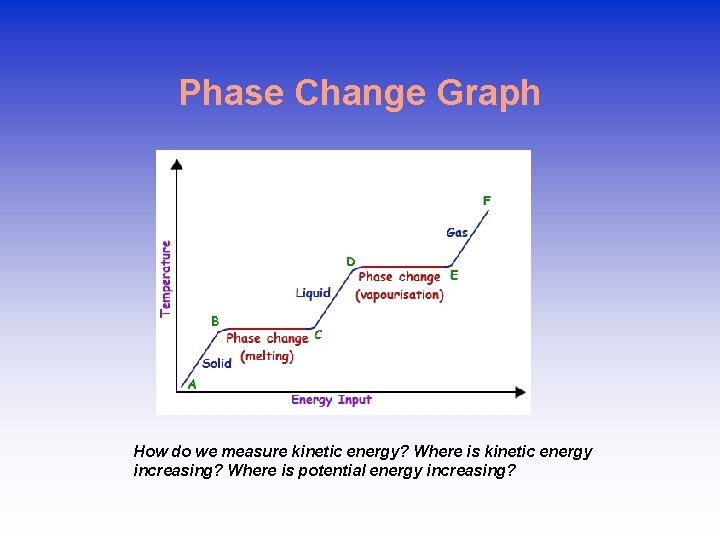
Phase Change Graph How do we measure kinetic energy? Where is kinetic energy increasing? Where is potential energy increasing?

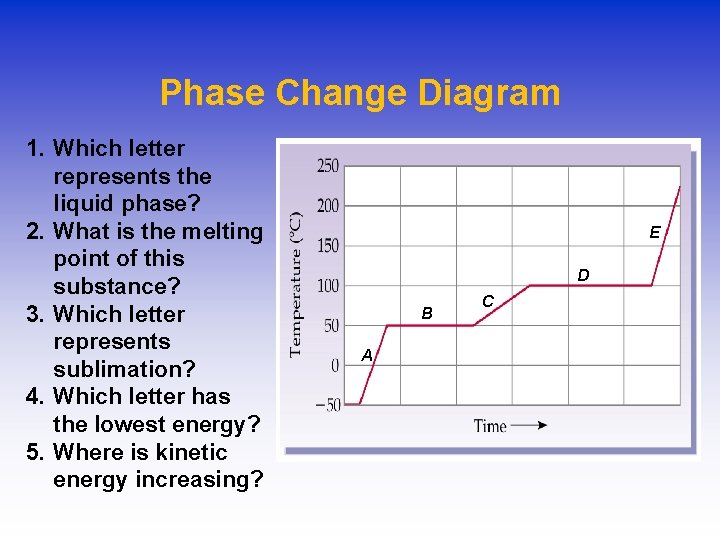
Phase Change Diagram 1. Which letter represents the liquid phase? 2. What is the melting point of this substance? 3. Which letter represents sublimation? 4. Which letter has the lowest energy? 5. Where is kinetic energy increasing? E D B A C

Density • • • Density is mass per unit volume D= m/V Density is measured in units of g/cm 3 or g/m. L Density is a physical property How would you determine the density of a metal?

You must show your work on your notes in order to be able to use them on the Note Check! D=m/V 1. What is the density of an object that weighs 4. 0 g and has a volume of 1. 0 m. L? • 4. 0 g/m. L 2. What is the mass of an object with a density of 0. 99 g/m. L and a volume of 12 m. L? • 11. 88 g = 12 g 3. What is the volume of an object with a density of 1. 23 g/m. L and a mass of 50. 0 g? • 40. 7 m. L

Physical Properties What are some physical properties? • Color • Melting and boiling point • Odor • Shape • Density • Phase of matter

Physical Changes – can be observed without changing the identity of the substance Some physical changes would be • boiling of a liquid • melting of a solid • dissolving a solid in a liquid to give a homogeneous mixture — a SOLUTION. • Phase changes are always physical

Chemical Properties • A property or characteristic of a substance that is observed during a reaction in which the chemical composition or identity of the substance is changed. – – – Heat of combustion Flammability Toxicity Reactivity Rusting

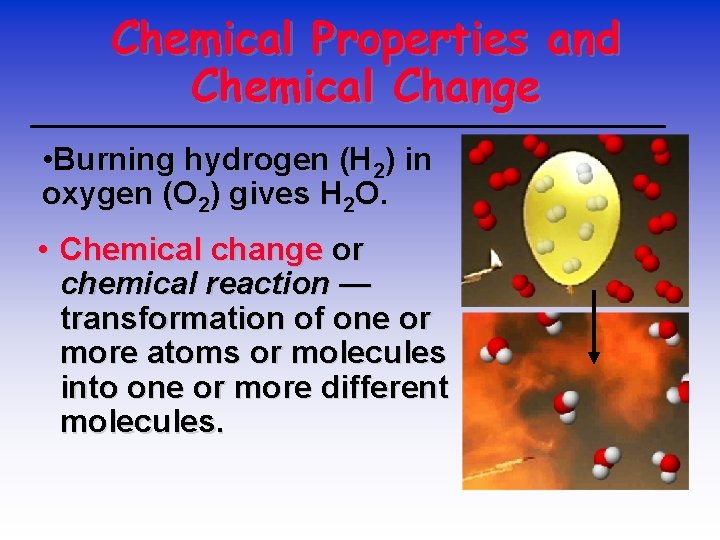
Chemical Properties and Chemical Change • Burning hydrogen (H 2) in oxygen (O 2) gives H 2 O. • Chemical change or chemical reaction — transformation of one or more atoms or molecules into one or more different molecules.

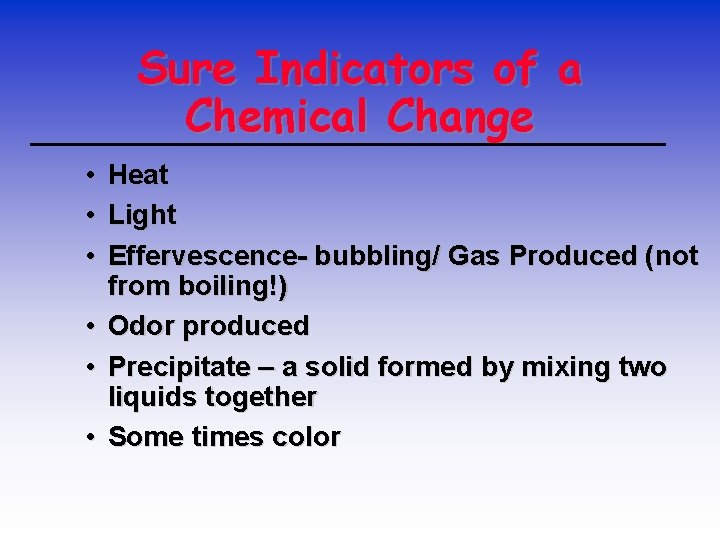
Sure Indicators of a Chemical Change • Heat • Light • Effervescence- bubbling/ Gas Produced (not from boiling!) • Odor produced • Precipitate – a solid formed by mixing two liquids together • Some times color

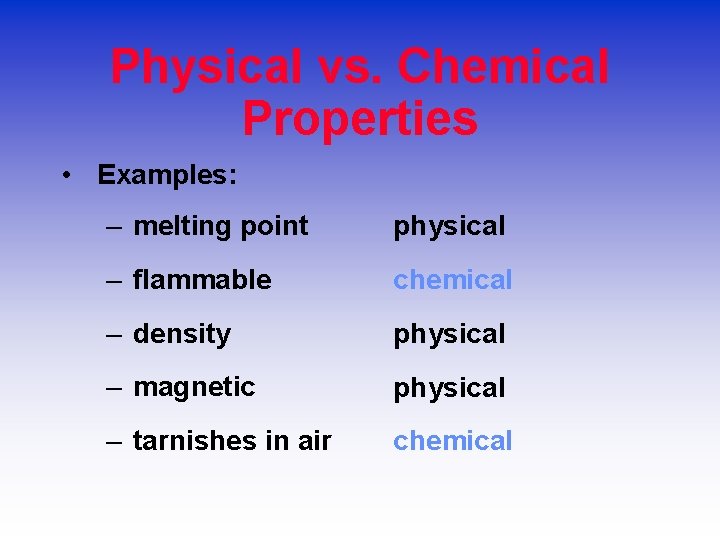
Physical vs. Chemical Properties • Examples: – melting point physical – flammable chemical – density physical – magnetic physical – tarnishes in air chemical

Physical vs. Chemical Changes • Examples: – rusting iron chemical – dissolving in water physical – burning a log chemical – melting ice physical – grinding spices physical

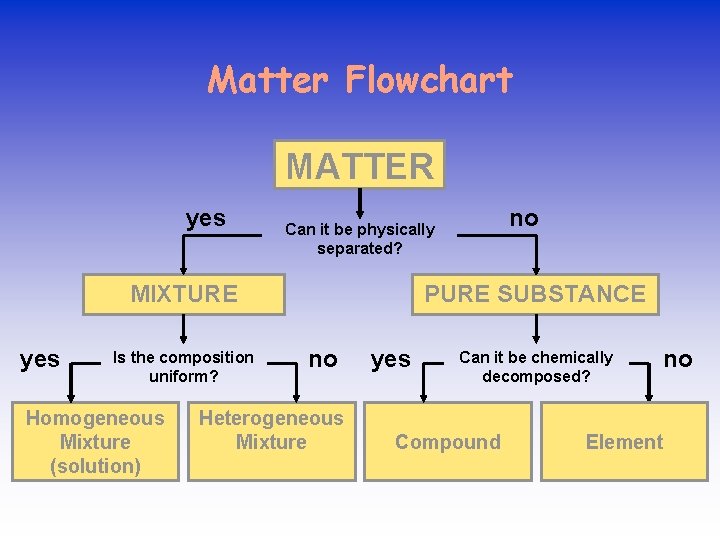
Matter Flowchart MATTER yes MIXTURE yes Is the composition uniform? Homogeneous Mixture (solution) no Can it be physically separated? PURE SUBSTANCE no Heterogeneous Mixture yes Can it be chemically decomposed? Compound no Element

Elements and Compounds • A pure substance- a substance with constant composition; a pure element or a pure compound • An element- a substance that cannot be decomposed into simpler substances by chemical or physical means. It consists of all of the same type of atoms. – Represented by elemental symbol • A compound- a substance made of two or more different elements chemically combined – Represented by a chemical formula – Always in definite proportions

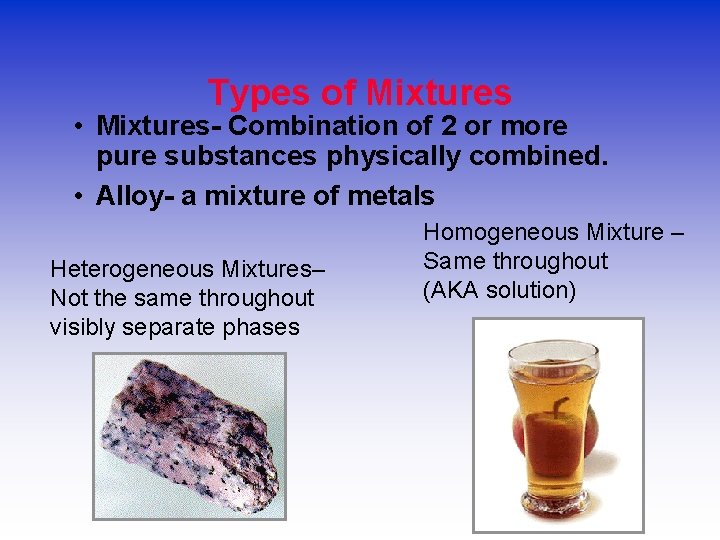
Types of Mixtures • Mixtures- Combination of 2 or more pure substances physically combined. • Alloy- a mixture of metals Heterogeneous Mixtures– Not the same throughout visibly separate phases Homogeneous Mixture – Same throughout (AKA solution)

Examples • Label the following as an element, compound, solution, alloy, or heterogeneous mixture 1. Solution 1. Lemonade 2. Compound 2. Sodium Chloride 3. Element 3. Gold 4. Alloy 4. Steel 5. Heterogeneous mixture 5. Pizza 6. Element 6. Sulfur 7. Heterogeneous mixture 7. Oil and water

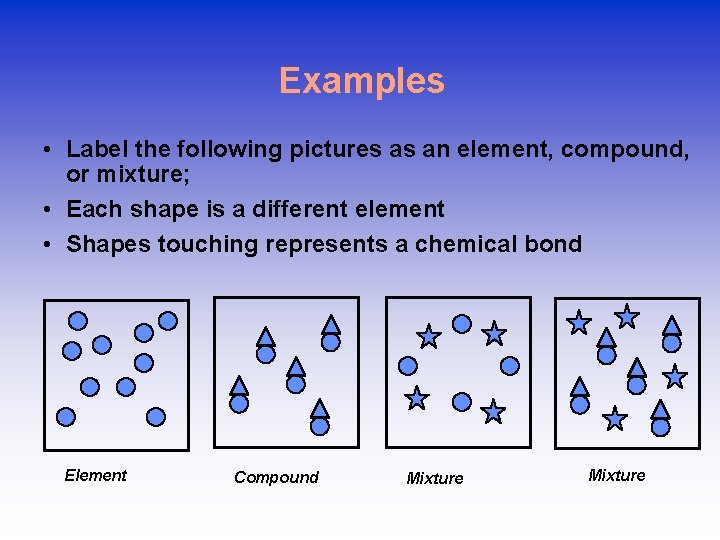
Examples • Label the following pictures as an element, compound, or mixture; • Each shape is a different element • Shapes touching represents a chemical bond Element Compound Mixture