Matter and Energy Essential Questions What is matter




















- Slides: 25

Matter and Energy Essential Questions: • What is matter? • How are various forms of energy different? • How are matter and energy related?

Text Book Definition of Chemistry: “Chemistry is the study of the composition of matter and the changes that matter undergoes. ” (Wilbraham, et al, 2005)

What is matter? • What makes up the world around us and the observable universe • Anything that has mass and volume

Mass is NOT weight! • Mass is a quantity of matter. It is the same everywhere! • Weight is a measure of the force with which gravity pulls on an object. Weight can change depending upon location.

Volume • A measure of the amount of space that an object takes up.

Density Example Problem 1 What is the mass of an object that has a density of 2 g/m. L and a volume of 4 m. L?

Density Example Problem 2 What is the volume of an object that has a density of 2 g/m. L and a mass of 4 g?

Matter can be identified by examining its properties. • Extensive Property: depends on the amount of matter in a sample • Examples: Mass and Volume

Matter can be identified by examining its properties. • Intensive Property: depends on the type of matter in a sample, not the amount • Every sample of a given substance has identical intensive properties because every sample has the same composition. • Examples: Hardness, Melting Point, Density…

Matter can be identified by examining its properties. • Physical properties are qualities or conditions of a substance that can be observed or measured without changing the substance’s composition • Describes the substance “being itself” • Examples: color, odor, boiling point, density, and solubility (how much can be dissolved)

Matter can be identified by examining its properties. • Chemical properties describe the ability of a substance to undergo a specific chemical change • They involve reactions, or changes in chemical composition • Examples: Hydrocarbons are combustible, Iron rusts

Physical vs. Chemical Change • Physical changes involve changes in some properties of a substance without changes in the composition • They involve different forms of the same substance. • Examples: melting, condensing, cutting, *dissolving

Physical vs. Chemical Change • Chemical changes involve changes in the composition of a substance (reactions) • One or more reactants form one or more different products • May include a transfer of energy (temp. change), change in color, formation of a gas, or formation of a precipitate • Examples: burning, combustion, rusting, oxidizing, rotting

Phases of Matter: Definite shape and definite volume Specific volume, but no specific shape (takes the shape of its container) Neither specific shape nor specific volume (takes shape and volume of its container)

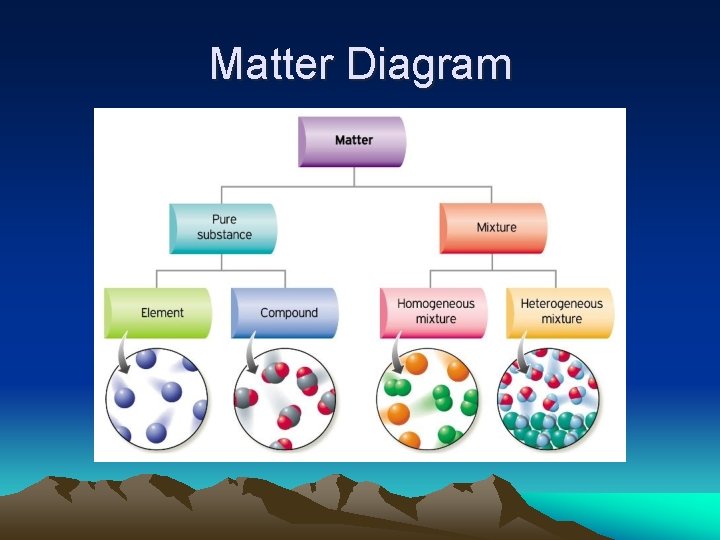
Classifications of Matter • Pure Substance: – a sample of matter that has a uniform and definite composition – Ex. element or compound

Classifications of Matter • Mixture: – a sample of matter that contains more than one component (substance) and that has a composition that can vary – Substances in a mixture have been physically mixed and can be separated physically

Classifications of Matter • Element: – simplest form of matter that has a unique set of properties – elements cannot be broken down (decomposed) by simple chemical means – Ex. hydrogen, oxygen, carbon, etc – *Diatomic Elements: HOFBr. INCl

Classifications of Matter • Compound: – a substance that contains 2 or more elements chemically combined in a fixed proportion – compounds can be broken down (decomposed) by simple chemical means – Ex. water (H 2 O), carbon dioxide (CO 2)

Types of Mixtures • Homogeneous Mixture: – the components of the mixture have been evenly mixed so the composition is uniform throughout – one “phase” – the composition can vary from mixture to mixture (sweet Kool-Aid vs. watereddown Kool-Aid) – a true solution is a homogeneous mixture

Types of Mixtures • Heterogeneous Mixture: – the components of the mixture have been unevenly mixed so the mixture is layered or clumpy – more than one “phase” – the composition is not uniform throughout – Ex. oil and water

Matter Diagram

Separating Mixtures • Components of a mixture can be separated physically by several different techniques: – Filtration: separates solids from a liquid (*will not remove dissolve substances from water)

Separating Mixtures – Distillation: separates components of a mixture based on differences in boiling point (the component with low bp evaporates and can be condensed and collected)

Separating Mixtures – Chromatography: separates components based on differences in attraction (charge differences) between a mobile phase and a stationary phase

Separating Mixtures • Centrifugation: separates components of a mixture based on differences in density