MATTER AND ENERGY CHAPTER TWO Concepts Matter consists






























- Slides: 49

MATTER AND ENERGY CHAPTER TWO

Concepts • Matter consists of elements and compounds, which in turn are made up of atoms, ions, or molecules • Whenever matter undergoes a physical or chemical change, no atoms are created or destroyed (the law of conservation of matter)

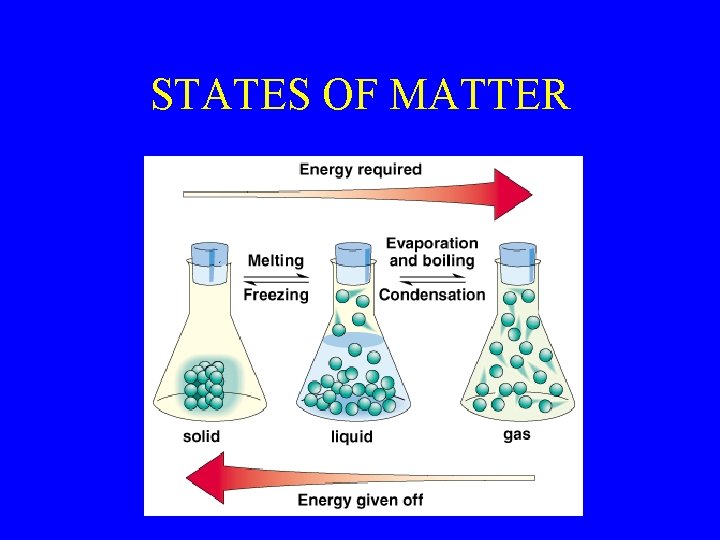
What is matter? • Three physical states: – Solid – Liquid – Gas • Two chemical forms of matter: – Elements – Compounds

STATES OF MATTER

Other facts about matter: • The smallest unit of matter is ___? • What is an ion? • What is a molecule and how are they held together? • What are subscripts and what do they represent? • The three physical states of matter on earth are____.

Building Blocks of Matter • Atoms (most basic) • Molecules • Ions

What is an element? • A fundamental type of matter that has a unique set of properties and cannot be broken down into simpler substances by chemical means • Periodic table: elements arranged based on their chemical behavior

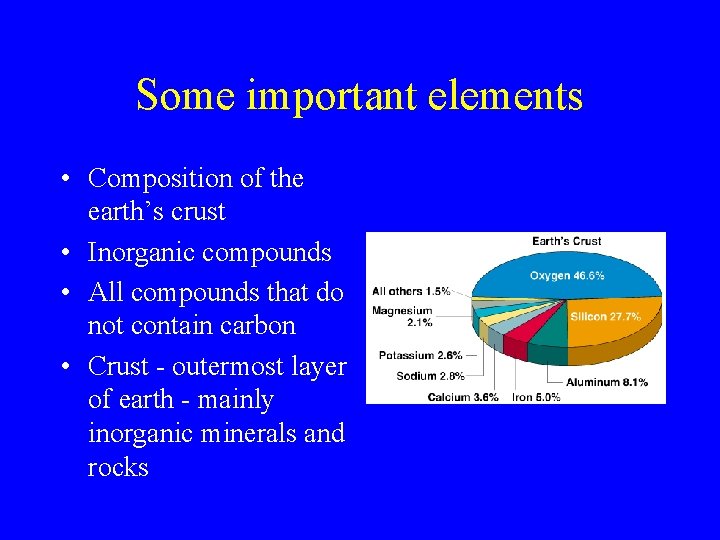
Some important elements • Composition of the earth’s crust • Inorganic compounds • All compounds that do not contain carbon • Crust - outermost layer of earth - mainly inorganic minerals and rocks

Atomic Theory • All elements are made of atoms • Most widely accepted scientific theory in chemistry

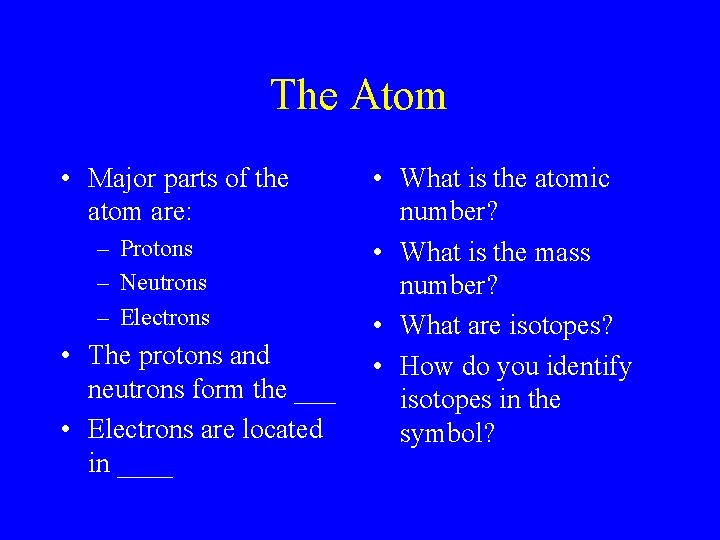
The Atom • Major parts of the atom are: – Protons – Neutrons – Electrons • The protons and neutrons form the ___ • Electrons are located in ____ • What is the atomic number? • What is the mass number? • What are isotopes? • How do you identify isotopes in the symbol?

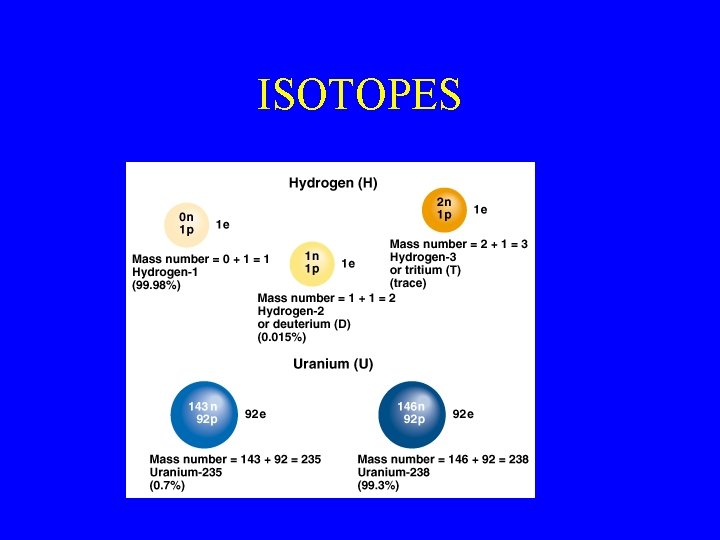
ISOTOPES

Molecule • A second building block of matter • Combination of two or more atoms of the same or different elements held together by chemical bonds • Basic building blocks of any compound

WHAT ARE IONS? • IF AN ATOM HAS 11 PROTONS AND 10 ELECTRONS IT IS A ______ION. • IF IT HAS 17 PROTONS AND 18 ELECTRONS IT IS A ___ ION. • HOW ARE THE CHARGES ON AN ION SHOWN AFTER THE SYMBOL?

Holding atoms together • What does a chemical formula tell you? • What are the characteristics of ionic bonds? • What is an example of an ionic bond? • What are covalent bonds? • What is an example of a covalent bond? • What are hydrogen bonds? • What is an example of a hydrogen bond compound?

Covalent bonds

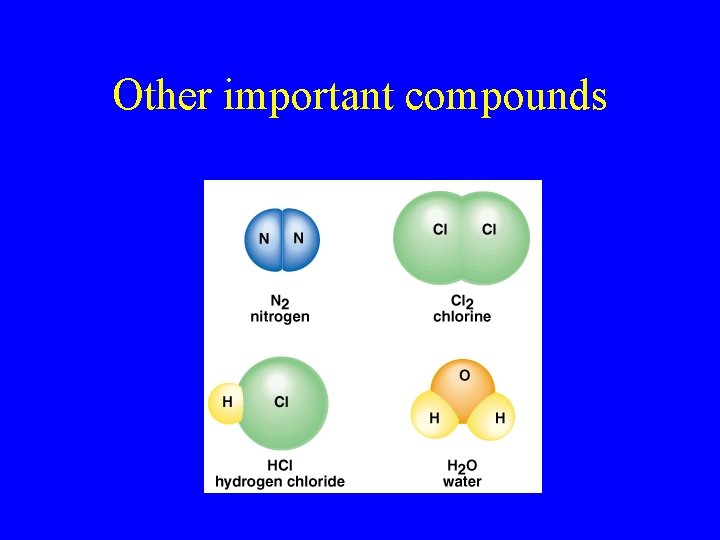
Other important compounds

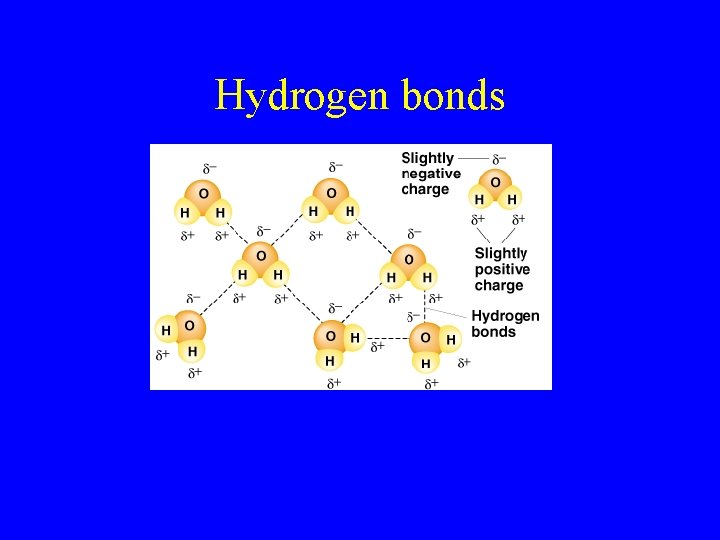
Hydrogen bonds

What are organic compounds? • What element do all organic compounds contain? What other elements can be also combined? • Organic compounds can be natural or synthetic. • Most organic compounds are covalent bonds.

P 38 • Organic Molecules – Monomers – Polymers

Types of organic compounds: • Hydrocarbons made up • Monomers of ___ • Polymers • Chlorinated • Complex hydrocarbons carbohydrates – An example would be: • Proteins • Chlorofluorocarbons - • Nucleic acids – An example would be: • Simple carbohydrates

MORE ON PROTEINS: • ALPHA-AMINO ACIDS - 20 DIFFERENT MONOMERS - # & SEQUENCE SPECIFIED BY GENETIC CODE IN DNA MOLECULES IN CELLS • NUCLEIC ACIDS DNA & RNA MADE BY LINKING MONOMERS CALLED NUCLEOTIDES TOGETHER

• GENES SEUQENCES OF NUCLEOTIDES CARRIES A CODE WHICH CONTAINS TRAITS PASSED FROM PARENTS TO OFFSPRING • GENOME - ALL OF THE GENETIC INFORMATION FOR AN ORGANISM. • What are GENE MUTATIONS? • What are CHROMOSOMES?

Matter quality • A measure of how useful a matter resource is - based on availability and concentration • High quality matter organized, concentrated and usually found near earth’s surface • Low quality disorganized, dilute, often deep underground or dispersed in the ocean or atmosphere - have little potential use as a matter resource.

Matter Quality

WHAT IS ENERGY? • • What is ENERGY ? What is WORK? What is a FORCE? Forms of energy - light, heat, electricity, chemical energy, mechanical energy, and nuclear energy

Types of energy • What is Kinetic energy? • What does it depend on? • Examples: wind, flowing water, electricity, electromagnetic radiation, heat, temperature • What is Potential energy? • What does it depend on? • Potential energy changes into kinetic energy etc.

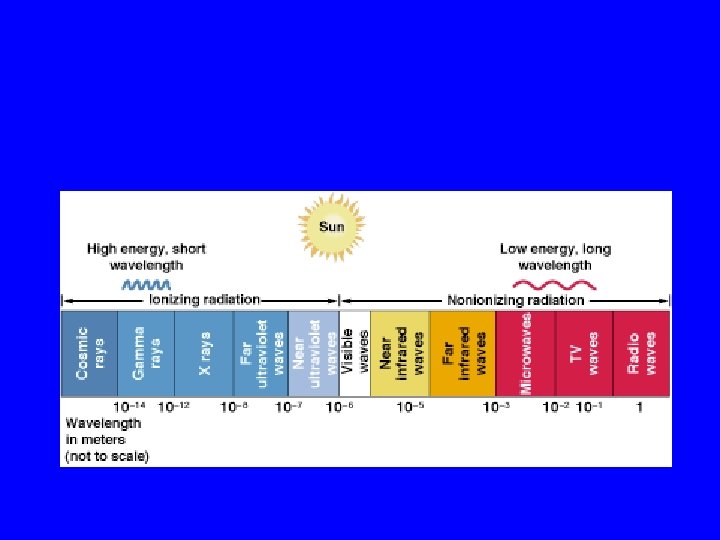
Electromagnetic spectrum • Transverse waves • Different wavelengths and frequencies • Ionizing radiation - harmful forms of electromagnetic radiation • Non-ionizing radiation - does not contain enough energy to form ions


Temperature • What is temperature? – The average speed of the motion of the molecules in a given sample of matter • What is heat? – The total kinetic energy of all the moving molecules within a given substance

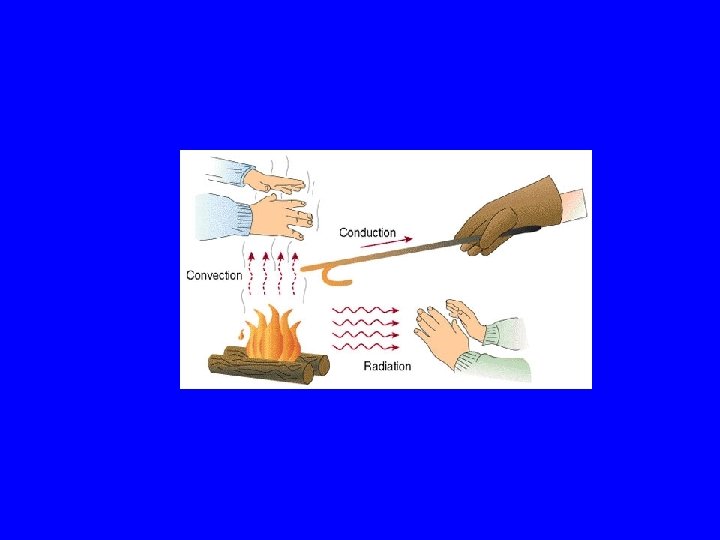
How is heat transferred? • What is convection? • What is Conduction? • What is Radiation?


Energy quality • An energy source’s ability to do useful work • High-quality - organized or concentrated can perform useful work – Electricity, coal, gasoline, sunlight, uranium • Low - quality - disorganized or dispersedcan perform little useful work – Heat in water, air, etc.

Changes in Matter • Physical • Chemical • Nuclear

Changes in matter • What is a Physical change? • What are some examples of physical changes? • All changes involve energy - taken in or released • What is a Chemical change ? • What is an example? • What is a CHEMICAL Equation? • Reactants --> products

Law of Conservation of Matter • All the matter on earth is here and cannot be “thrown away” - there is no “away” • Earth is a closed system • Matter cannot be created nor destroyed • Matter is not consumed

Nuclear changes • Natural radioactivity- when nuclei of certain isotopes spontaneously break down into one or more different isotopes – Three types: – 1. Natural radioactive decay – 2. Nuclear fission – 3. Nuclear fusion

Law of Conservation of matter and energy • Applies to nuclear changes because a certain amount of mass (matter) is changed into energy. • The TOTAL amount of matter and energy involved remains the same

What are Pollutants? • Severity of their effects depends on: – Chemical nature – Concentration – Persistence • Three categories: – Degradable – nonpersistent – Biodegradable – Slowly degradable • Presistent – Nondegradable • �

Nuclear Changes • Changes in the nucleus of the atom • Three types: – Natural radioactive decay – Nuclear fission – Nuclear fusion

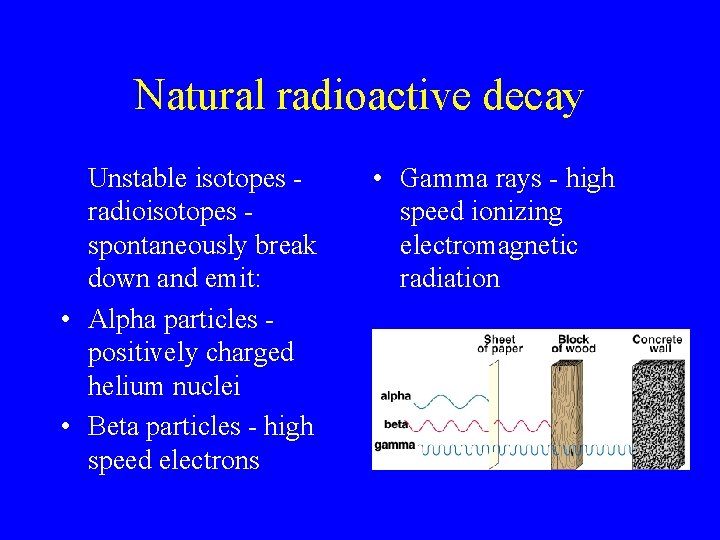
Natural radioactive decay Unstable isotopes radioisotopes spontaneously break down and emit: • Alpha particles positively charged helium nuclei • Beta particles - high speed electrons • Gamma rays - high speed ionizing electromagnetic radiation

Half-life • Rate of decay • Time needed for one half of the nuclei in a radioisotope to decay and emit their radiation • Eventually forms a new element • Is not affected by temp. pressure, chemical changes, etc. • Rule is store for 10 half-lives for safety

Nuclear fission • Nuclei of atoms with large mass numbers are split into lighter nuclei • Neutrons used to split • Releases more neutrons and energy • Critical mass - needed to start reaction

More on fission • Atomic bombs uncontrolled nuclear fission • Damage cells • Used in nuclear power plants

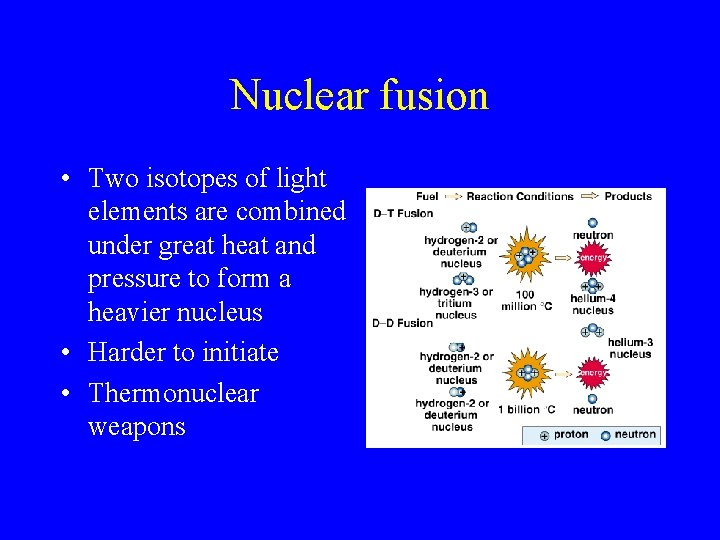
Nuclear fusion • Two isotopes of light elements are combined under great heat and pressure to form a heavier nucleus • Harder to initiate • Thermonuclear weapons

• Ionizing Radiation – from natural or background sources – Can come from space, soil, food, etc. – Has energy to knock electrons from atoms • Can disrupt living cells, interfere with body processes and cause cancer. – Nonionizing radiation doesn not contain enough energy to form ions.

First law of thermodynamics • In all physical and chemical changes, energy is neither created nor destroyed but it may be converted from one form to another • Energy input always equals energy output • You can’t get something for nothing cannot get more energy out of a system than is put in!!!

Second law of thermodynamics • When energy is changed from one form to another, some useful energy is always degraded to lower quality less useful energy usually heat lost to the environment • We ALWAYS end up with less useful energy than we started with. • An incandescent light bulb - 5 % light, 95% heat

More on 2 nd law • We can NEVER recycle or reuse high quality energy to do useful work. • You get high quality matter and energy in your body, you use it and you add low quality waste matter and heat to the environment.

What is energy efficiency? • A measure of how much useful work is accomplished by a particular input of energy into a system • Always measured as a percent (%) • Affects life because you get and use high quality matter and energy , use it and add low quality heat and waste back into the environment.