Matter and Energy Chapter Outline Classification of Matters





















- Slides: 63

Matter and Energy

Chapter Outline • • • Classification of Matters Physical/Chemical Properties Physical/Chemical Changes Energy Temperature, Heat, and Specific Heat 2

In Your Room • Everything you can see, touch, smell or taste in your room is made of matter. • Chemists study the differences in matter and how that relates to the structure of matter. 3

What is Matter? • Matter: anything that occupies space and has mass • Matter is actually composed of a lot of tiny little pieces: Atoms and Molecules 4

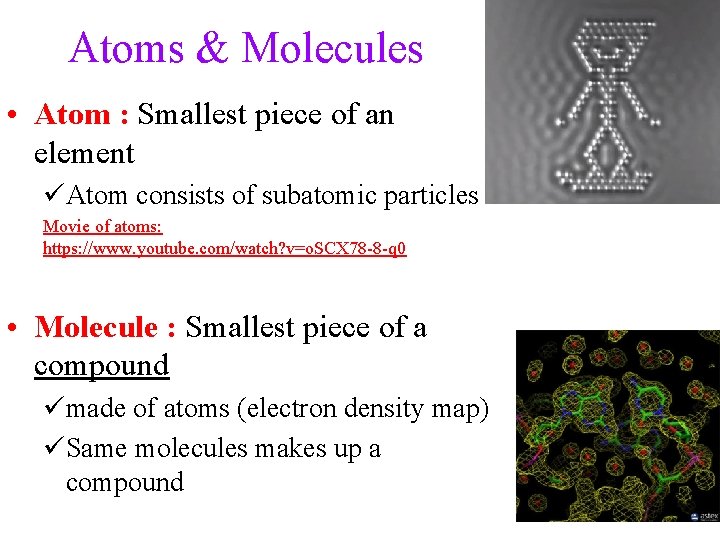
Atoms & Molecules • Atom : Smallest piece of an element üAtom consists of subatomic particles Movie of atoms: https: //www. youtube. com/watch? v=o. SCX 78 -8 -q 0 • Molecule : Smallest piece of a compound ümade of atoms (electron density map) üSame molecules makes up a compound 5

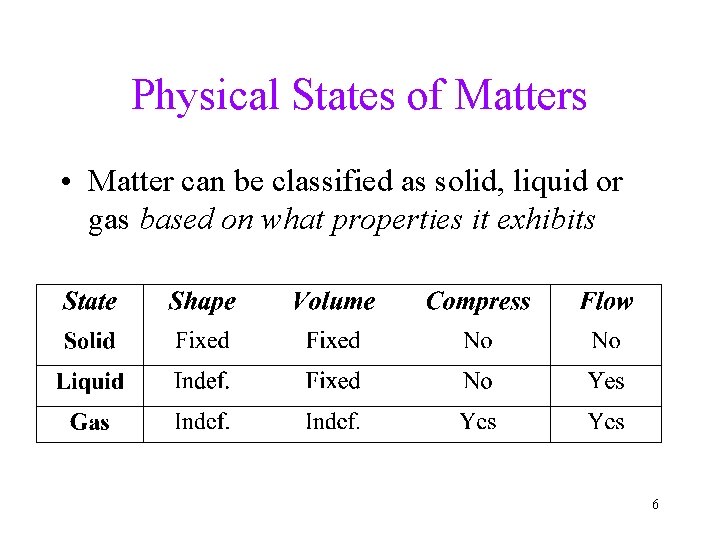
Physical States of Matters • Matter can be classified as solid, liquid or gas based on what properties it exhibits 6

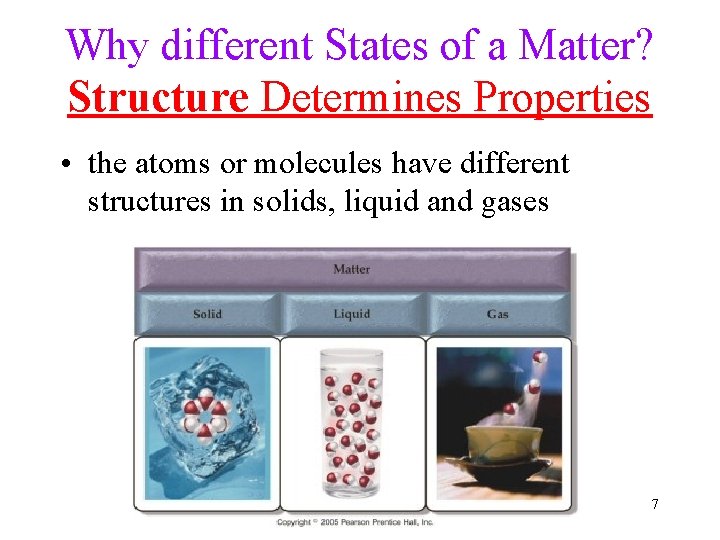
Why different States of a Matter? Structure Determines Properties • the atoms or molecules have different structures in solids, liquid and gases 7

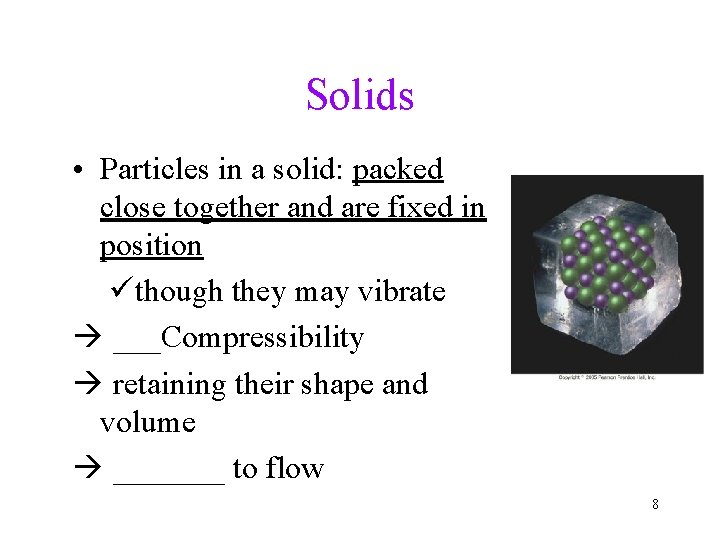
Solids • Particles in a solid: packed close together and are fixed in position üthough they may vibrate ___Compressibility retaining their shape and volume _______ to flow 8

Liquids • Particles are closely packed, but they have some ability to move around _____ compressibility ______ to flow, yet not to escape and expand to fill the container (not “antigravity”) 9

Gases • The particles have complete freedom from each other (not sticky to each other) • The particles are constantly flying around, bumping into each other and the container • There is a lot of empty space between the particles (low density) Compressible Able to flow and Fill space (“antigravity”) 10

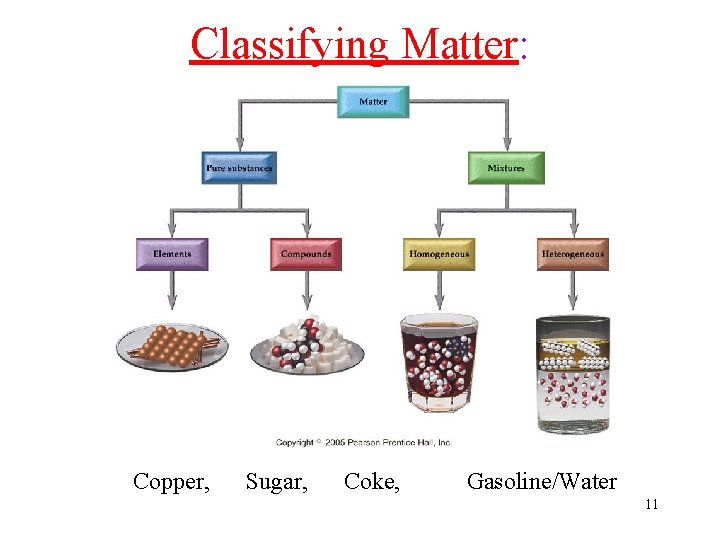
Classifying Matter: Copper, Sugar, Coke, Gasoline/Water 11

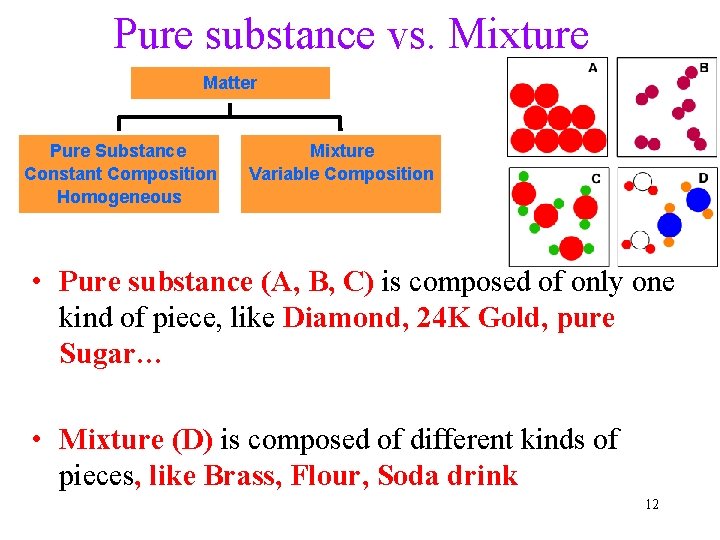
Pure substance vs. Mixture Matter Pure Substance Constant Composition Homogeneous Mixture Variable Composition • Pure substance (A, B, C) is composed of only one kind of piece, like Diamond, 24 K Gold, pure Sugar… • Mixture (D) is composed of different kinds of pieces, like Brass, Flour, Soda drink 12

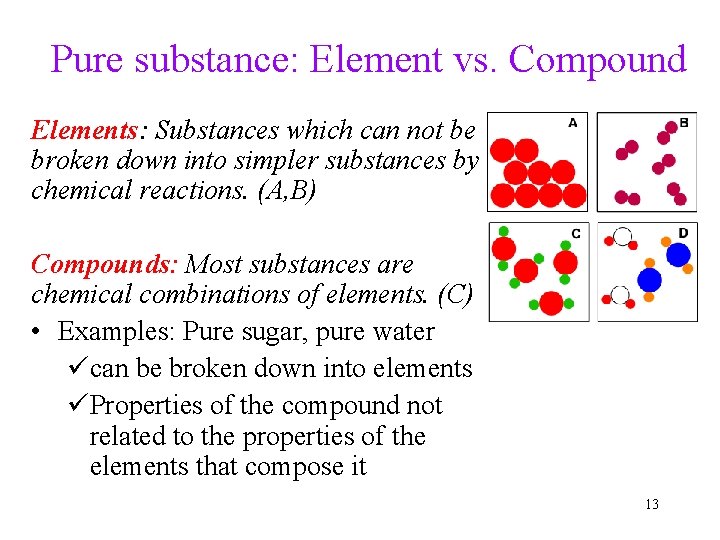
Pure substance: Element vs. Compound Elements: Substances which can not be broken down into simpler substances by chemical reactions. (A, B) Compounds: Most substances are chemical combinations of elements. (C) • Examples: Pure sugar, pure water ücan be broken down into elements üProperties of the compound not related to the properties of the elements that compose it 13

Elements • 116 known, 91 are found in nature ü others are man-made • Abundance = percentage found in nature ü Hydrogen: most abundant in the universe (fuel for starlight, such as in the Sun) ü Oxygen: most abundant element (by mass) on earth and in the human body ü Silicon: abundant on earth surface • every sample of an element is made up of lots of identical atoms 14

15

Compounds • Composed of elements in fixed percentages üwater is 89% O & 11% H • billions of known compounds • Organic (sugar, glycerol) or inorganic (table salt) • same elements can form more than one different compound üwater and hydrogen peroxide contain just hydrogen and oxygen ücarbohydrates all contain just C, H & O (sugar, starch, glucose) 16

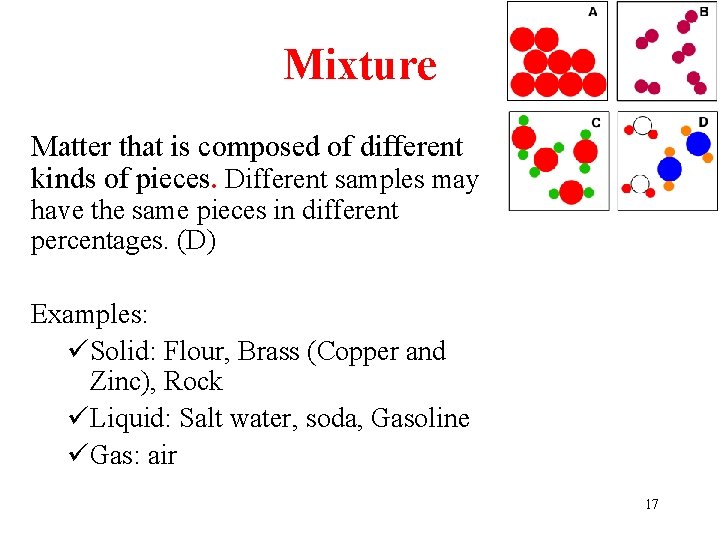
Mixture Matter that is composed of different kinds of pieces. Different samples may have the same pieces in different percentages. (D) Examples: üSolid: Flour, Brass (Copper and Zinc), Rock üLiquid: Salt water, soda, Gasoline üGas: air 17

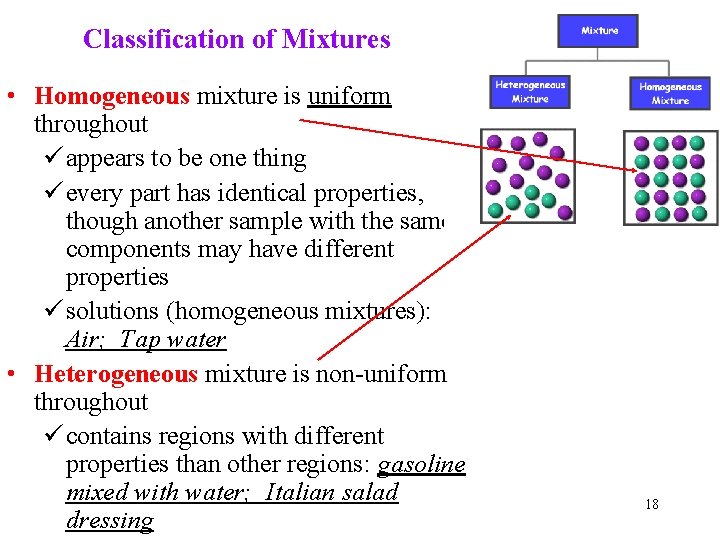
Classification of Mixtures • Homogeneous mixture is uniform throughout ü appears to be one thing ü every part has identical properties, though another sample with the same components may have different properties ü solutions (homogeneous mixtures): Air; Tap water • Heterogeneous mixture is non-uniform throughout ü contains regions with different properties than other regions: gasoline mixed with water; Italian salad dressing 18

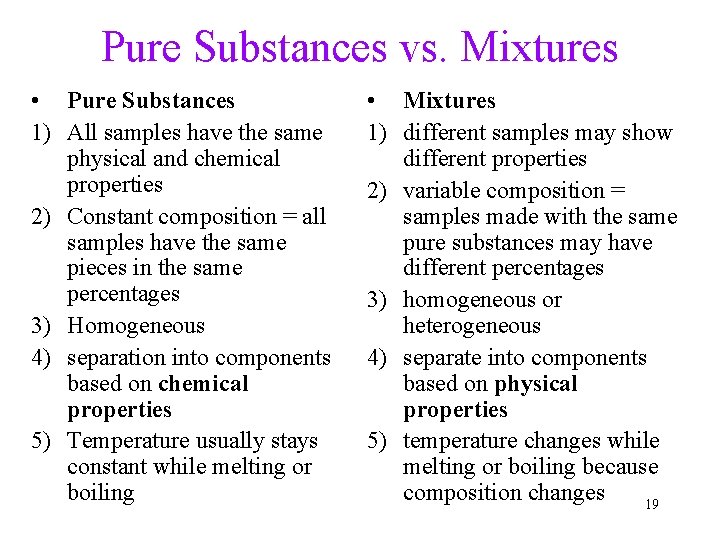
Pure Substances vs. Mixtures • Pure Substances 1) All samples have the same physical and chemical properties 2) Constant composition = all samples have the same pieces in the same percentages 3) Homogeneous 4) separation into components based on chemical properties 5) Temperature usually stays constant while melting or boiling • Mixtures 1) different samples may show different properties 2) variable composition = samples made with the same pure substances may have different percentages 3) homogeneous or heterogeneous 4) separate into components based on physical properties 5) temperature changes while melting or boiling because composition changes 19

Classifying Matter in Daily Life Pure substance or Mixture: (a) Lead fishing weight (b) Tap water (c) Distilled water (d) Italian salad dressing (e) Silver dollar Pure element or compound? Mixture: Homo/Heterogeneous? (a) Pb, pure element (b) Mixture, homogenous (c) H 2 O, pure compound (d) Water + vinegar + oil, heterogeneous (e) Ag, pure 20

How Matters Differ from each other? • Physical Properties are the characteristics of matter that can be changed without changing its composition ücharacteristics that are directly observable • Chemical Properties are the characteristics that determine how the composition of matter changes as a result of contact with other matter or the influence of energy ücharacteristics that describe the behavior of matter 21

Some Physical Properties 22

Some Chemical Properties 23

Iron: Physical Properties vs. Chemical Properties • silvery solid at room • easily corroded in moist air temperature with a metallic to form brownish rust taste and smooth texture • melts at 1538°C and boils at • when added to hydrochloric acid, it disappears and 4428°C • Density 7. 87 g/cm 3 generates hydrogen gas • Can be magnetized • more reactive than silver, • conducts electricity but less reactive than • thermal conductivity magnesium 24

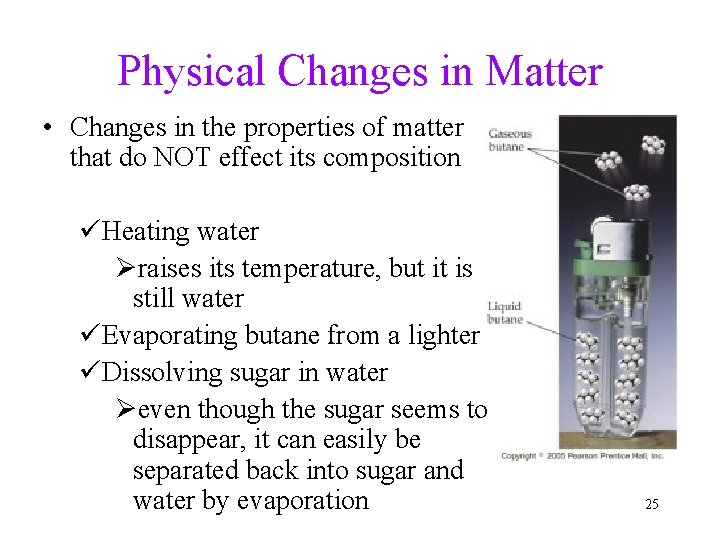
Physical Changes in Matter • Changes in the properties of matter that do NOT effect its composition üHeating water Øraises its temperature, but it is still water üEvaporating butane from a lighter üDissolving sugar in water Øeven though the sugar seems to disappear, it can easily be separated back into sugar and water by evaporation 25

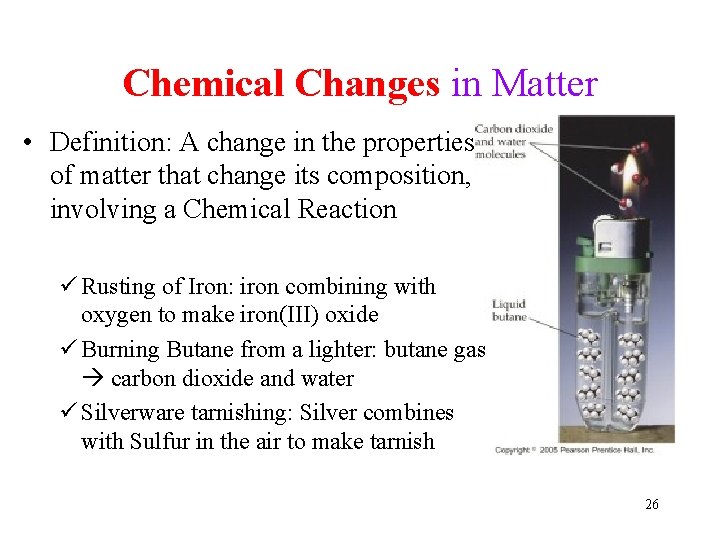
Chemical Changes in Matter • Definition: A change in the properties of matter that change its composition, involving a Chemical Reaction ü Rusting of Iron: iron combining with oxygen to make iron(III) oxide ü Burning Butane from a lighter: butane gas carbon dioxide and water ü Silverware tarnishing: Silver combines with Sulfur in the air to make tarnish 26

Is it a Physical or Chemical Change? • a physical change results in a different form of the same substance ü the kinds of molecules don’t change • a chemical change results in one or more completely new substances ü the new substances have different molecules than the original substances ü you will observe different physical properties because the new substances have their own physical properties 27

Phase Changes: Physical Changes • • Boiling = liquid to gas Melting = solid to liquid Subliming = solid to gas Condensing = gas to liquid Freezing = liquid to solid Deposition = gas to solid state changes require heating or cooling the substance ü evaporation is not a simple phase change, it is a solution process 28

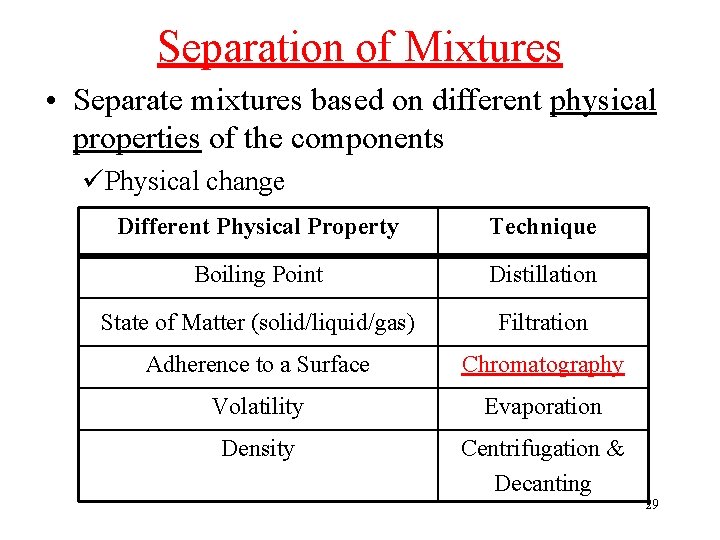
Separation of Mixtures • Separate mixtures based on different physical properties of the components üPhysical change Different Physical Property Technique Boiling Point Distillation State of Matter (solid/liquid/gas) Filtration Adherence to a Surface Chromatography Volatility Evaporation Density Centrifugation & Decanting 29

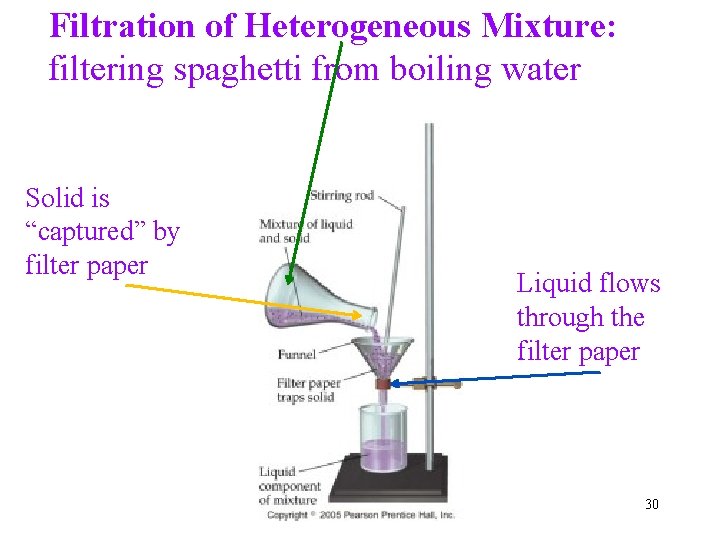
Filtration of Heterogeneous Mixture: filtering spaghetti from boiling water Solid is “captured” by filter paper Liquid flows through the filter paper 30

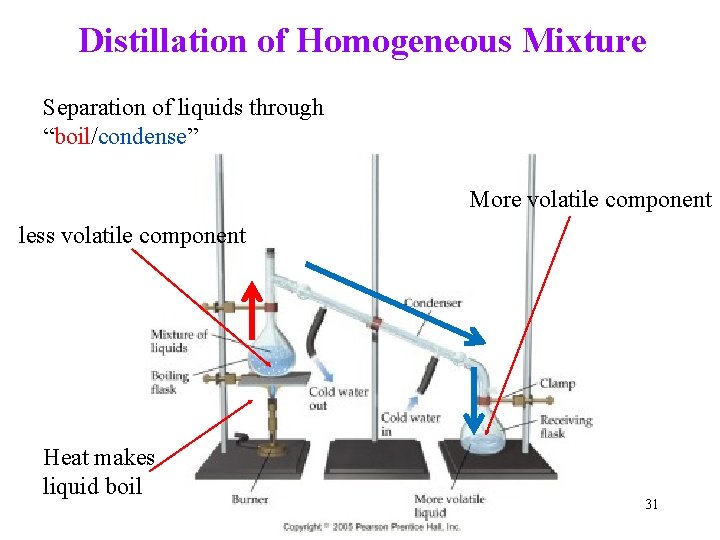
Distillation of Homogeneous Mixture Separation of liquids through “boil/condense” More volatile component less volatile component Heat makes liquid boil 31

Law of Conservation of Mass • Antoine Lavoisier: “Father of Modern Chemistry”: “Matter is neither created nor destroyed in a chemical reaction” • Massbefore = Massafter • Massreactants = Massproducts 32

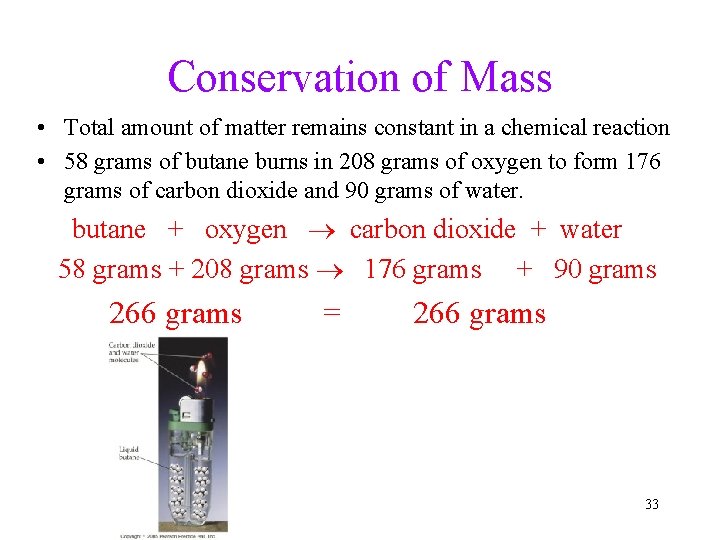
Conservation of Mass • Total amount of matter remains constant in a chemical reaction • 58 grams of butane burns in 208 grams of oxygen to form 176 grams of carbon dioxide and 90 grams of water. butane + oxygen carbon dioxide + water 58 grams + 208 grams 176 grams + 90 grams 266 grams = 266 grams 33

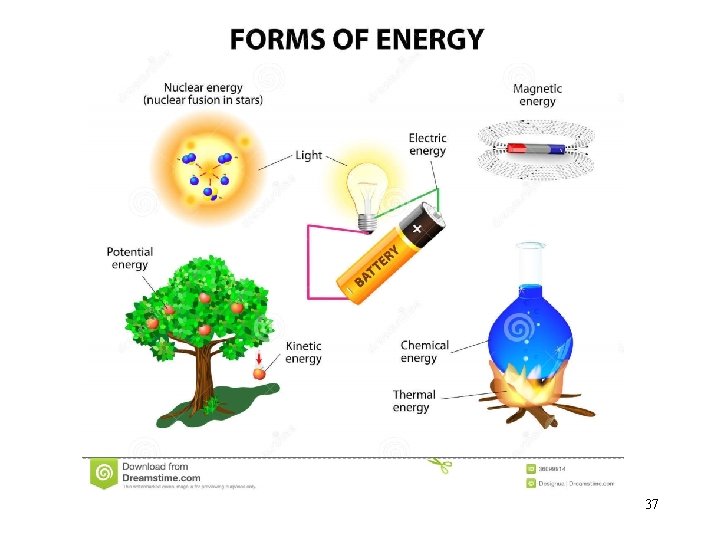
Energy do not have mass and volume (<-> Matter) • Energy is anything that has the capacity to do work • Chemistry is the study of matter, Chemical process involve energy change • it can cause physical and/or chemical changes in matter 34

Matter Possesses Energy • when a piece of matter possesses energy, it can give some or all of it to another object • all chemical and physical changes result in the matter changing energy 35

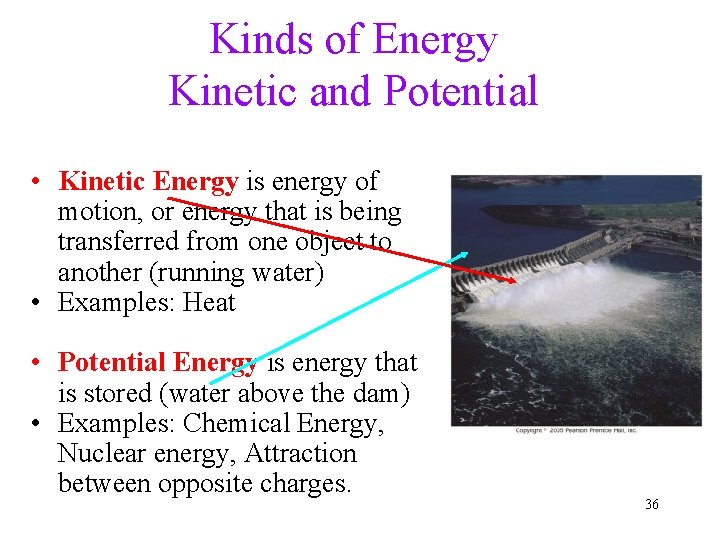
Kinds of Energy Kinetic and Potential • Kinetic Energy is energy of motion, or energy that is being transferred from one object to another (running water) • Examples: Heat • Potential Energy is energy that is stored (water above the dam) • Examples: Chemical Energy, Nuclear energy, Attraction between opposite charges. 36

37

Law of Conservation of Energy • “Energy can neither be created nor destroyed” • the total amount of energy in the universe is constant – there is no process that can increase or decrease that amount We can transfer energy from one place to another: Solar energy from the Sun to the Earth We can transform the energy: • Solar panel: Solar energy ______ • Gas engine: Chem energy _______ • Applying the brake: Kinetic energy _______ 38

Units of Energy • calorie (cal) is the amount of energy needed to raise one gram of water by 1°C ükcal = energy needed to raise 1000 g of water 1°C üfood Calories = kcals Energy Conversion Factors 1 calorie (cal) 1 Calorie (Cal) 1 kilowatt-hour (k. Wh) = = = 4. 184 joules (J) 1000 calories (cal) 3. 60 x 106 joules (J) 39

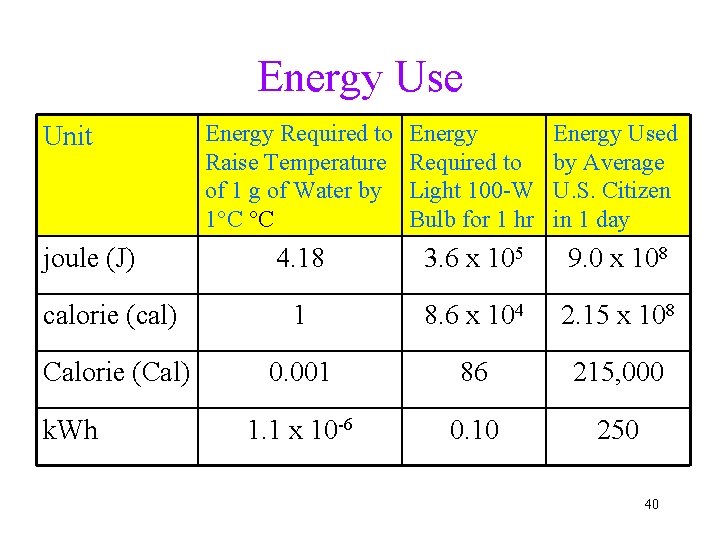
Energy Use Energy Required to Raise Temperature of 1 g of Water by 1°C °C Energy Required to Light 100 -W Bulb for 1 hr Energy Used by Average U. S. Citizen in 1 day 4. 18 3. 6 x 105 9. 0 x 108 calorie (cal) 1 8. 6 x 104 2. 15 x 108 Calorie (Cal) 0. 001 86 215, 000 1. 1 x 10 -6 0. 10 250 Unit joule (J) k. Wh 40

Conversion Problem: 955 Cal Joule? Information Given: 955 Cal Find: ? J Conv. Fact. 1 cal = 4. 184 Joules 3. 44 x 103 J 41

What is Heat • Heat is the exchange of thermal energy between samples of matter How Heat flows? • Heat transfers from matter that has _______ temperature to matter that has ______ temperature üuntil they reach the same temperature • heat is exchanged through molecular collisions between two samples 42

Exothermic/Endothermic Processes • Physical/Chemical changes are often accompanied by energy change • Exothermic: A change _______ heat. Example: Combustion (burning); Condensation of water • Endothermic: A change ____ heat. Example: Ice melting; Evaporation of alcohol 43

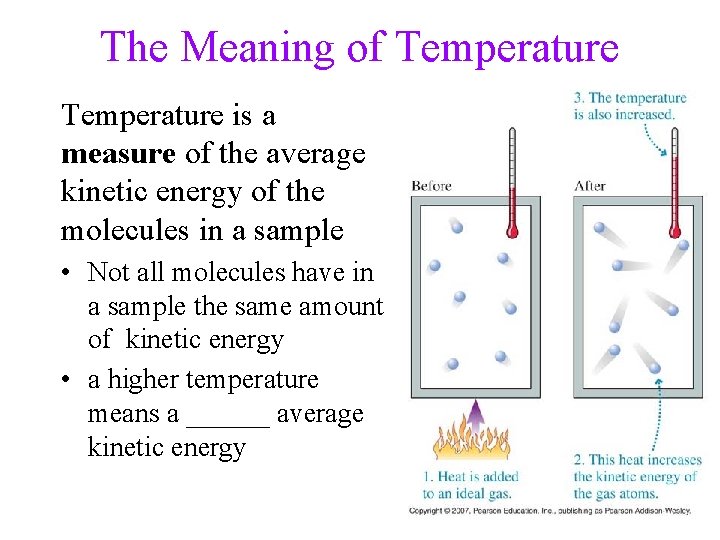
The Meaning of Temperature is a measure of the average kinetic energy of the molecules in a sample • Not all molecules have in a sample the same amount of kinetic energy • a higher temperature means a ______ average kinetic energy 44

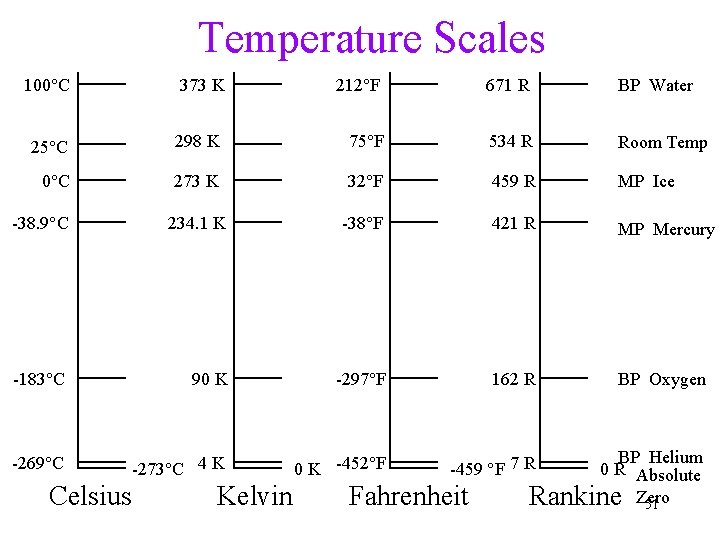
Fahrenheit Temperature Scale Two reference points: • Freezing point of concentrated saltwater (0°F) • Average body temperature (100°F) ümore accurate measure now set average body temperature at 98. 6°F • Room temperature is about 75°F 45

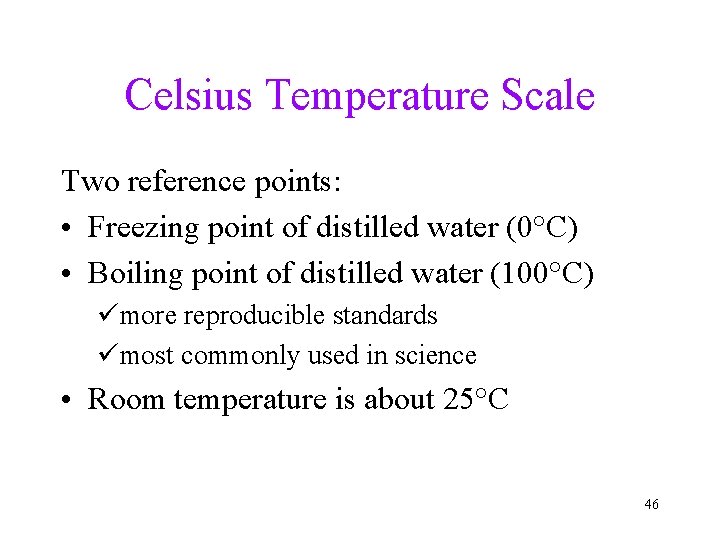
Celsius Temperature Scale Two reference points: • Freezing point of distilled water (0°C) • Boiling point of distilled water (100°C) ümore reproducible standards ümost commonly used in science • Room temperature is about 25°C 46

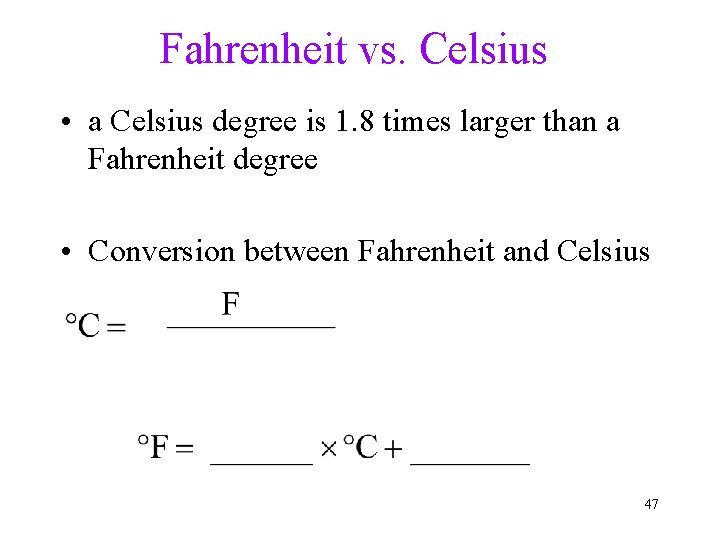
Fahrenheit vs. Celsius • a Celsius degree is 1. 8 times larger than a Fahrenheit degree • Conversion between Fahrenheit and Celsius 47

The Kelvin Temperature Scale • Kelvin scale is an absolute scale, meaning it measures the actual temperature of an object • 0 K = Absolute Zero: all molecular motion would stop • 0 K is theoretically the lowest temperature in the universe ü 0 K = -273°C = -459°F üAbsolute Zero is a theoretical value 48

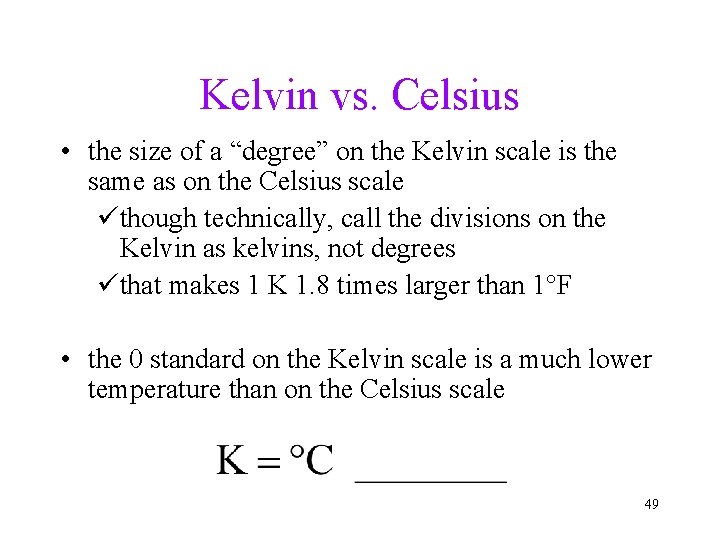
Kelvin vs. Celsius • the size of a “degree” on the Kelvin scale is the same as on the Celsius scale üthough technically, call the divisions on the Kelvin as kelvins, not degrees üthat makes 1 K 1. 8 times larger than 1°F • the 0 standard on the Kelvin scale is a much lower temperature than on the Celsius scale 49

Extremes of Temperature On the Earth, • Lowest temperature recorded: -89. 2°C (-128. 6 °F, 184 K) • Highest air temperature recorded: ~60°C In science lab, • the highest temperature: 4 x 1012 K (? ) • the lowest temperature: ~10 -10 K (? ) 50

Temperature Scales 100°C 373 K 212°F 671 R BP Water 25°C 298 K 75°F 534 R Room Temp 0°C 273 K 32°F 459 R MP Ice -38. 9°C 234. 1 K -38°F 421 R MP Mercury -183°C 90 K -297°F 162 R BP Oxygen -269°C -273°C 4 K 0 K -452°F -459 °F 7 R Celsius Kelvin Fahrenheit BP Helium 0 R Absolute Rankine Zero 51

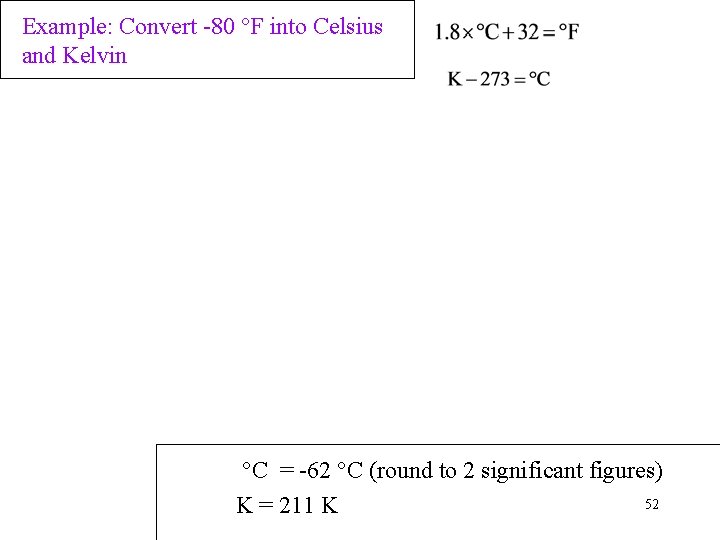
Example: Convert -80 °F into Celsius and Kelvin °C = -62 °C (round to 2 significant figures) 52 K = 211 K

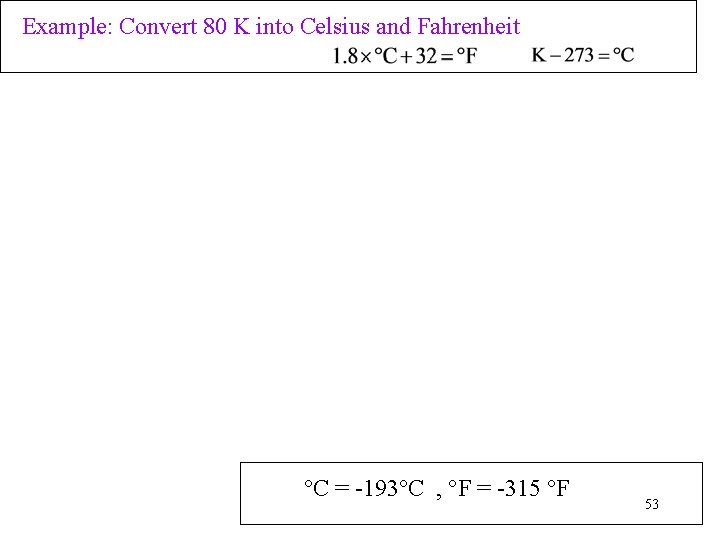
Example: Convert 80 K into Celsius and Fahrenheit °C = -193°C , °F = -315 °F 53

Heat and Temperature • The temperature increase of an object (DT) depends on the amount of heat energy added (q). When heating a pot of water, the more heat is given, the _____ (higher / lower) temperature increase (aka larger DT) • For the same amount of heat q, the temperature increase of an object DT depends on its mass (m). Using the same heat, ______ (small / large) pot of water will have higher temperature change (aka, larger DT). 54

Heat and Temperature • Given the same heat, the temperature increase of an object (DT) depends on what the material it is made of. ü Same amount of heat upon 1 kg of gold vs. 1 kg of aluminum: gold will be heated to higher temperature. What makes different materials differ from each other is the Specific Heat. 55

Specific Heat: A Physical Property • Specific heat c = heat absorbed by 1 gram of the substance to raise the temperature by 1 °C • c = q / (m DT) ücal/g°C or J/g°C üwaters specific heat = 4. 184 J/g°C for liquid Øor 1. 000 cal/g°C Øless for ice and steam 56

About Specific Heat • Specific heat is a property of the type of matter ü it can be used to identify the type of matter • Higher specific heat, more heat needed to increase temperature • water’s high specific heat a good cooling agent ü it absorbs a lot of heat for a relatively small mass • Heavier atoms tend to have lower specific heat. 57

What difference does Specific Heat make? • Diamond and graphite are both made from pure carbon. From 0 C to 100 C, which object absorbs more heat, 1. 0 kg diamond or 1. 0 kg graphite? • Given 100. J heat, which object will have higher temperature change, 1. 0 kg silver or 1. 0 kg aluminum? 58

Heat Gain or Loss by an Object Heat energy gained or lost by an object depends on • how much material there is • what the material is, and • how much the temperature changed The sign of temperature change and calculated heat • If T > 0 (temperature increase), q > 0 is for heat absorbed (gained) • If T < 0, q < 0 is for heat released (lost) 59

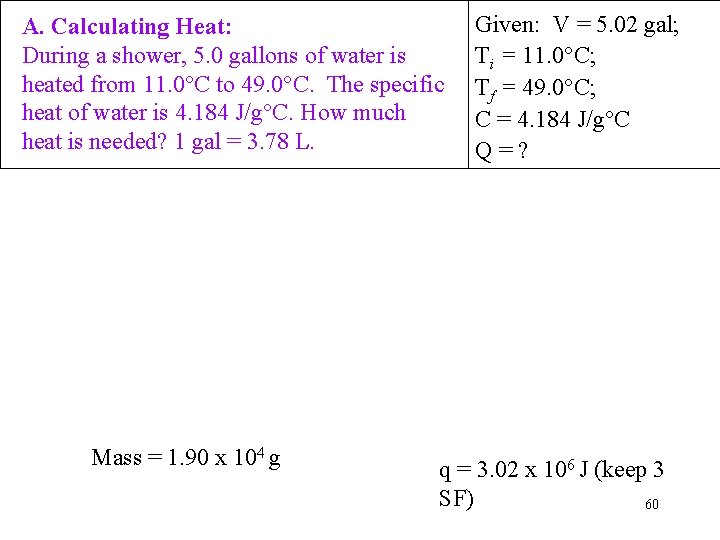
A. Calculating Heat: During a shower, 5. 0 gallons of water is heated from 11. 0°C to 49. 0°C. The specific heat of water is 4. 184 J/g°C. How much heat is needed? 1 gal = 3. 78 L. Mass = 1. 90 x 104 g Given: V = 5. 02 gal; Ti = 11. 0°C; Tf = 49. 0°C; C = 4. 184 J/g°C Q=? q = 3. 02 x 106 J (keep 3 SF) 60

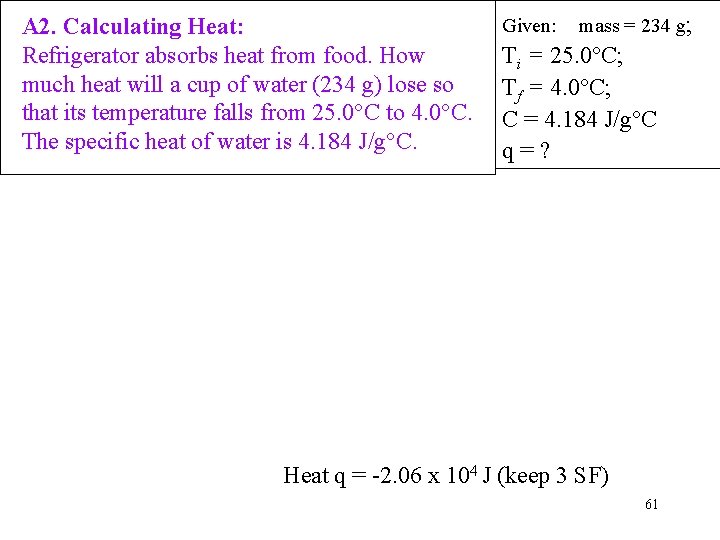
A 2. Calculating Heat: Refrigerator absorbs heat from food. How much heat will a cup of water (234 g) lose so that its temperature falls from 25. 0°C to 4. 0°C. The specific heat of water is 4. 184 J/g°C. Given: mass = 234 g; Ti = 25. 0°C; Tf = 4. 0°C; C = 4. 184 J/g°C q=? Heat q = -2. 06 x 104 J (keep 3 SF) 61

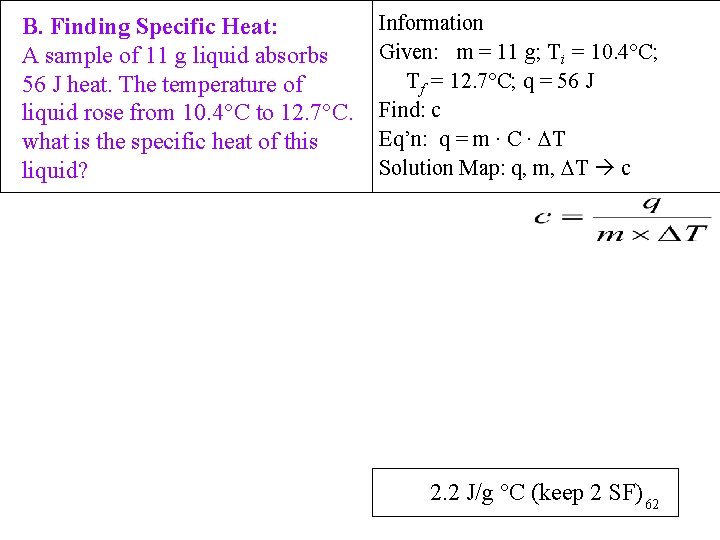
B. Finding Specific Heat: A sample of 11 g liquid absorbs 56 J heat. The temperature of liquid rose from 10. 4°C to 12. 7°C. what is the specific heat of this liquid? Information Given: m = 11 g; Ti = 10. 4°C; Tf = 12. 7°C; q = 56 J Find: c Eq’n: q = m ∙ C ∙ T Solution Map: q, m, T c 2. 2 J/g °C (keep 2 SF) 62

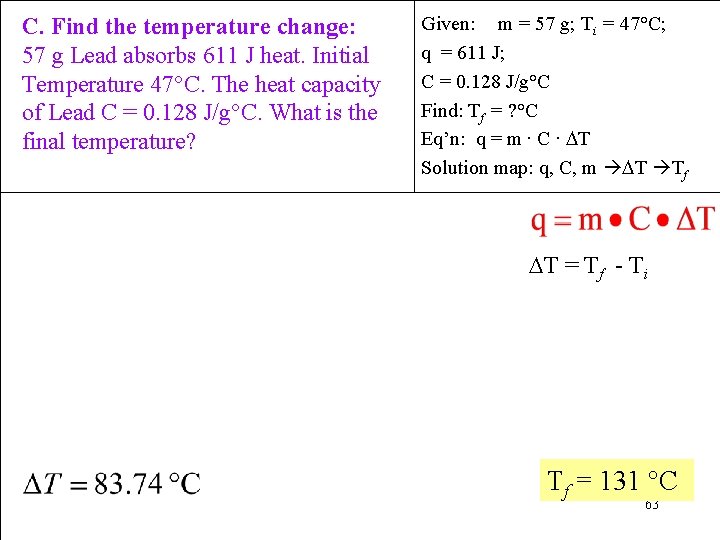
C. Find the temperature change: 57 g Lead absorbs 611 J heat. Initial Temperature 47°C. The heat capacity of Lead C = 0. 128 J/g°C. What is the final temperature? Given: m = 57 g; Ti = 47°C; q = 611 J; C = 0. 128 J/g°C Find: Tf = ? °C Eq’n: q = m ∙ C ∙ T Solution map: q, C, m T Tf T = Tf - Ti Tf = 131 °C 63