MATTER AND CHANGE Chemistry Introduction Chapter 2 Matter








- Slides: 12

MATTER AND CHANGE Chemistry Introduction Chapter 2 Matter: Anything that has mass and takes up space

Properties • Properties describe matter • They can be used to identify a substance • They can be used to classify matter

Properties of Matter • Extensive – Depends on amount in sample • Mass, volume • Intensive – depends on type of matter, not the amount • Hardness, density, color, conductivity

Classifying Matter Pure Substance • Element – all atoms the same • Examples: Iron, lead, carbon, calcium • Compound (two or more elements chemically combined) • Examples: Carbon dioxide, water, sodium chloride, magnesium oxide

Classifying Matter Mixture • Two or more substances physically combined • Examples: salt water, trail mix, air • Can be physically separated • Homogeneous: evenly distributed; uniform appearance • Solutions: Solute dissolved in solvent • Dye in water • Heterogeneous: distinct parts • Salad

States of Matter • Solid – definite shape and volume • Liquid – definite volume • Gas – no shape or volume, able to compress • Gas vs. Vapor: a gas is the term for something that is gaseous at room temperature; a vapor is something that is normally solid or liquid at room temperature, but can become a gas • What is an example of a gas? • What is an example of a vapor?

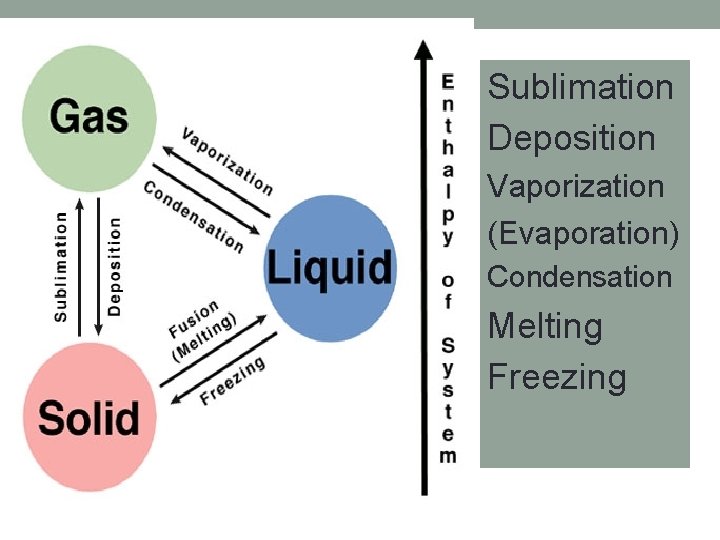
Phase changes Sublimation Deposition Vaporization (Evaporation) Condensation Melting Freezing

Properties of Matter • Physical property – a trait you can observe or measure without changing the substance • State, color, melting point, boiling point, density • Chemical property – the ability of a substance to undergo a specific chemical change • Ability to combust, reactivity, p. H

Physical changes • Any change that does not affect the composition • Can be reversible • Phase changes • Crushing • Breaking • Grinding Chemical • Changes what the matter is • Rearranges atoms • Not easily reversed • Burning • Rusting

Chemical Change Clues • Formation of precipitate (solid from two liquids) • Transfer of energy • Production of gas • Change in color

Law of Conservation of Mass • During any chemical reaction, the mass of the products is always equal to the mass of the reactants • Nothing can be created, nor destroyed

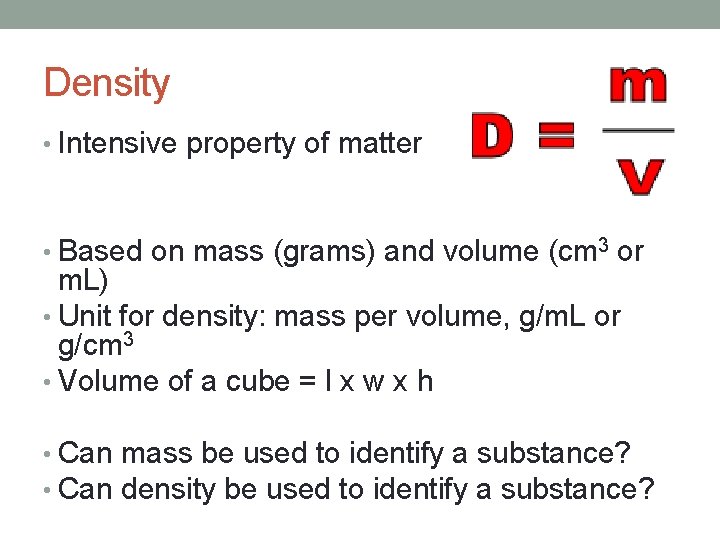
Density • Intensive property of matter • Based on mass (grams) and volume (cm 3 or m. L) • Unit for density: mass per volume, g/m. L or g/cm 3 • Volume of a cube = l x w x h • Can mass be used to identify a substance? • Can density be used to identify a substance?