Matter and Change 2 1 Properties of Matter














- Slides: 22

Matter and Change 2. 1 Properties of Matter

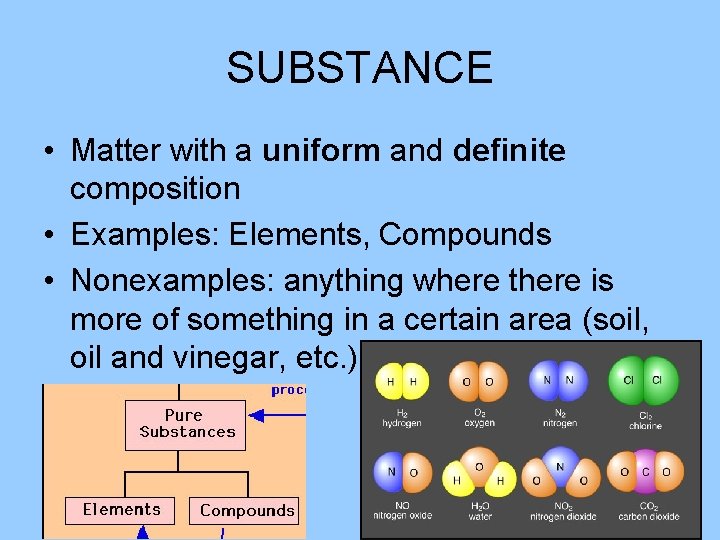
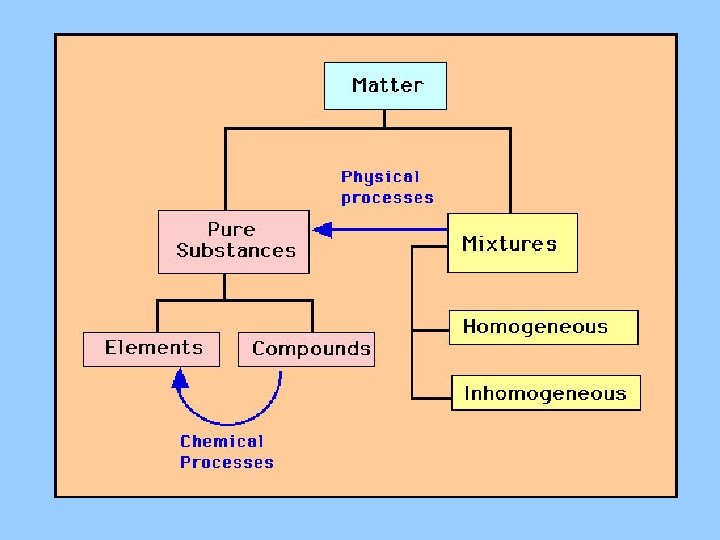
SUBSTANCE • Matter with a uniform and definite composition • Examples: Elements, Compounds • Nonexamples: anything where there is more of something in a certain area (soil, oil and vinegar, etc. )

PHYSICAL PROPERTY • A quality or condition that can be observed or measured without changing the substance’s composition. • Examples: state, color, melting/boiling point, etc.

PHYSICAL CHANGE • Reversible or Irreversible • Boil, freeze, melt, condense (ALL PHASE CHANGES) • Break, split, grind, cut, and crush, etc.

SOLIDS vs. LIQUIDS vs. GASES STATE Easy to Flows? Definite Compress? Volume? Shape? SOLID LIQUID GAS • MOVEMENT? Click

Vapor • A gas that is usually a solid or liquid at room temperature • We say “water VAPOR” because water is usually a liquid at room temperature

Chapter 2 2. 2 Mixtures

MIXTURE DEF. • Physical blend of two or more components • HOMOGENEOUS • HETEROGENEOUS

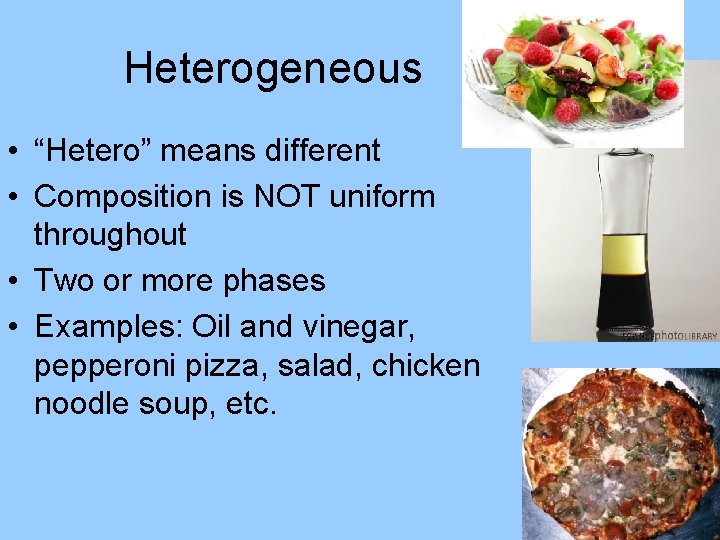
Heterogeneous • “Hetero” means different • Composition is NOT uniform throughout • Two or more phases • Examples: Oil and vinegar, pepperoni pizza, salad, chicken noodle soup, etc.

Homogeneous • • “Homo” means same Composition IS uniform throughout One phase Examples: Kool Aid, Vinegar and water, stainless steel, food coloring in water, etc. • (most are liquids)

You Try…

Chapter 2 2. 3 Elements and Compounds

Elements Vs. Compounds • Element: Simplest form of matter – Example: C, Al, Mg, Au, Na, etc. • Compound: More than one element chemically combined in a fixed proportion – Example: Na. Cl, H 2 O, K 3 N • Elements = symbol, Compound = Formula

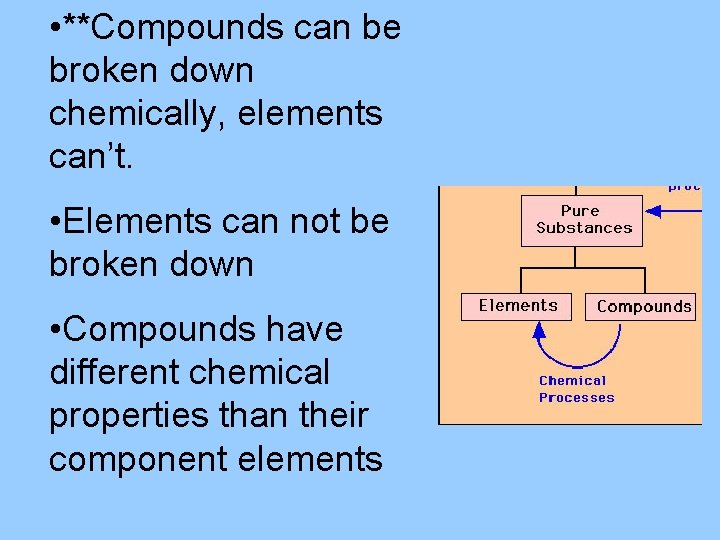
• **Compounds can be broken down chemically, elements can’t. • Elements can not be broken down • Compounds have different chemical properties than their component elements

Chemical Change • There is a new chemical composition after the change • Test composition before and after the change to determine if a chemical change has occurred!!!!


Chapter 2 2. 4 Chemical Reactions

Chemical Property • The ability of a substance to undergo a specific chemical change • ****Burn, rot, rust, decompose, ferment, explode, and corrode

Chemical Change • Composition of matter ALWAYS changes • Test composition before and after the change to determine if a chemical change has occurred!!!!

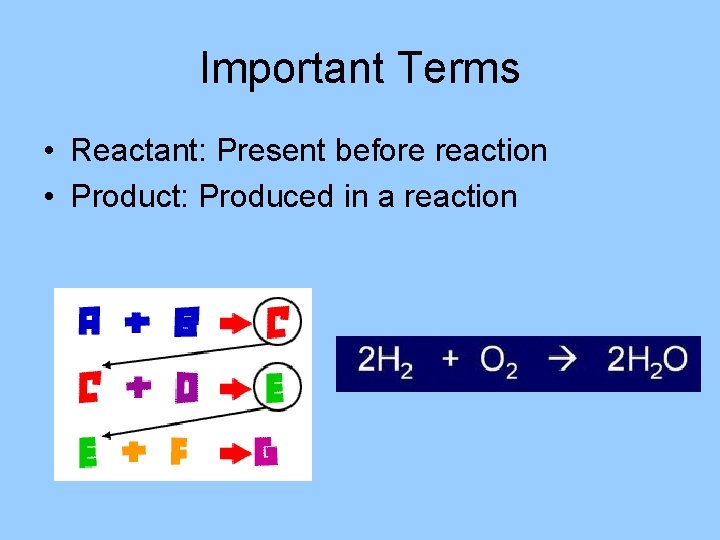
Important Terms • Reactant: Present before reaction • Product: Produced in a reaction

Clues • • Transfer of Energy Change in Color Production of Gas Formation of Precipitate

Law of Conservation of Mass • Mass of the products is always equal to the mass of the reactants • In physical AND chemical changes