Matter and Atomic Structure Text ref Ch 3

























- Slides: 42

Matter and Atomic Structure Text ref. Ch. 3, (pg. 52)

Contents What are elements? How atoms combine


3. 1: What Are Elements? Text ref. 52 -59

Objectives Describe the particles within atoms and the structure of atoms. Relate the energy levels of atoms to the chemical properties of elements. Define the concept of isotopes.

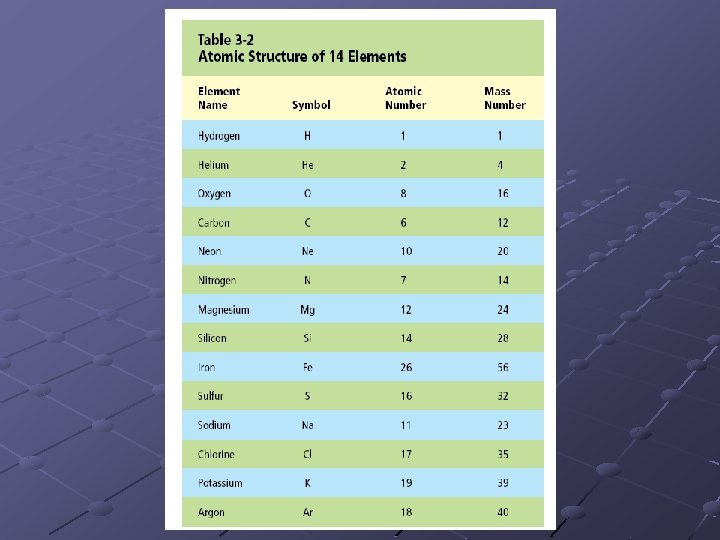
Elements Defined as a substance that cannot be broken down into simpler substances by physical or chemical means. 92 naturally occurring elements Elements are organized using the Periodic Table of Elements

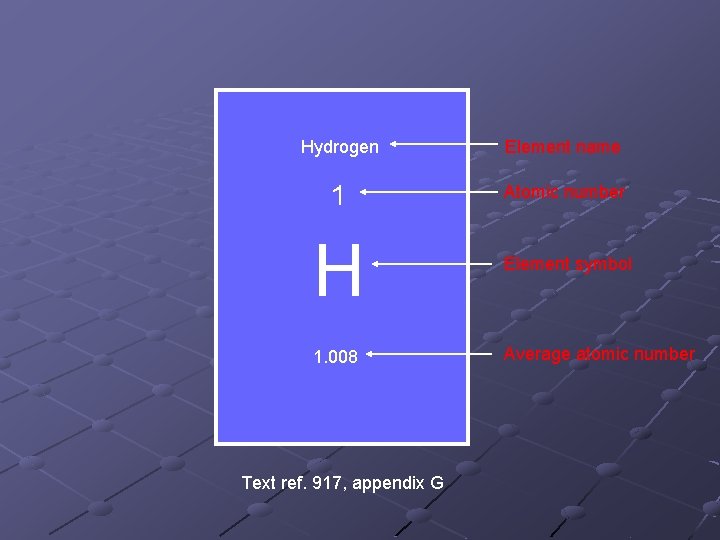
Hydrogen Element name 1 Atomic number H Element symbol 1. 008 Average atomic number Text ref. 917, appendix G




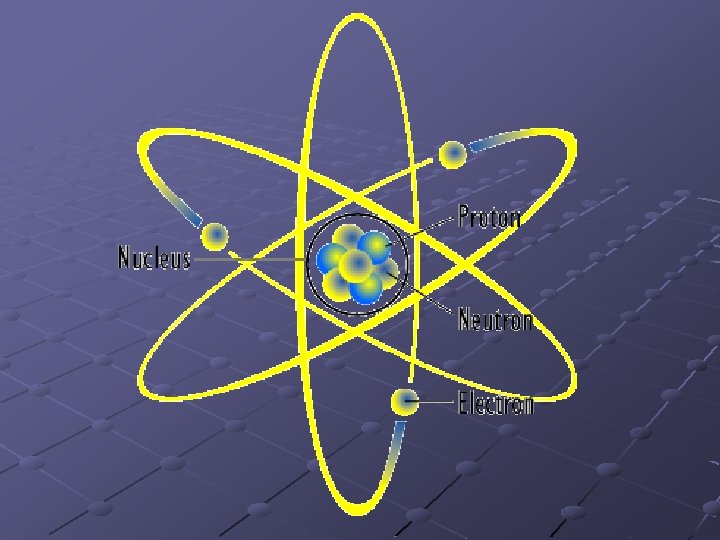
Elements and the Atom Smallest particle of an element that retains the properties and characteristics of that element. Composed of subatomic particles n n Nucleus: center or atom, composed of protons and neutrons Proton: tiny particle having a positive charge Neutron: particle having no charge, but about the same mass Electron: much smaller mass than proton or neutron, but has a negative charge equal to a proton


Electrons in Energy Levels Electrons occupy regions of space around the nucleus of an atom known as energy levels. Each energy level contains a number of orbitals. The number of electrons that may be found at an energy level can be detemined by the following formula. #electrons = 2 n 2

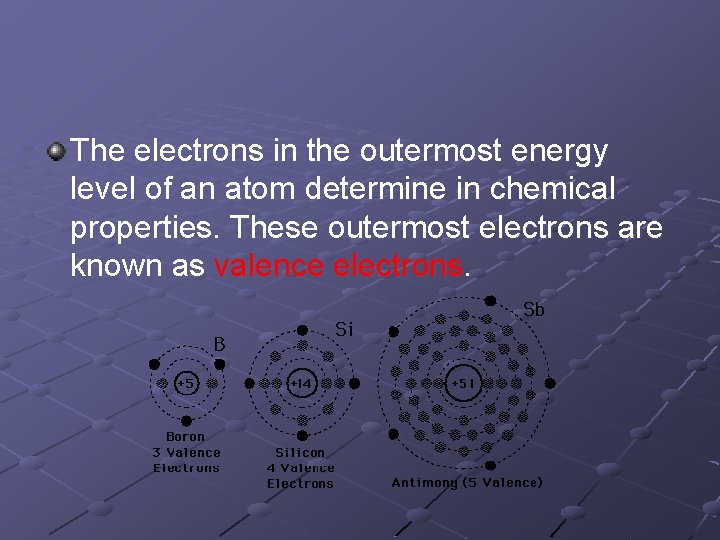
The electrons in the outermost energy level of an atom determine in chemical properties. These outermost electrons are known as valence electrons.

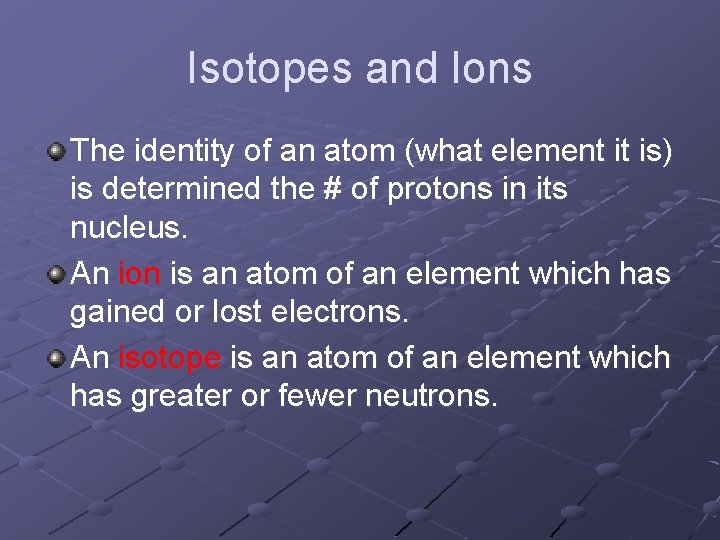
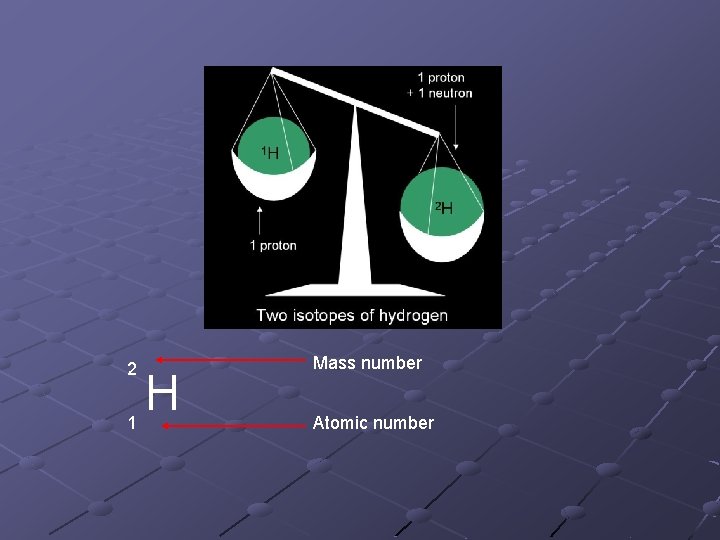
Isotopes and Ions The identity of an atom (what element it is) is determined the # of protons in its nucleus. An ion is an atom of an element which has gained or lost electrons. An isotope is an atom of an element which has greater or fewer neutrons.

2 1 H Mass number Atomic number


Radioactivity Nuclear decay as particles are lost in an atom leads to the emission of radiation. Types of radiation include… n n n Alpha: helium nucleus Beta: electron Gamma: high energy EM radiation


Elemental Abundances Abundance of Elements in the Universe Elements are not found in equal numbers in the universe or on Earth. Some are more common than others. Element Parts per million Hydrogen 739, 000 Helium 240, 000 Oxygen 10, 700 Carbon 4, 600 Neon 1, 340 Iron 1, 090 Nitrogen 950 Silicon 650 Magnesium 580 Sulfur 440 All Others 650

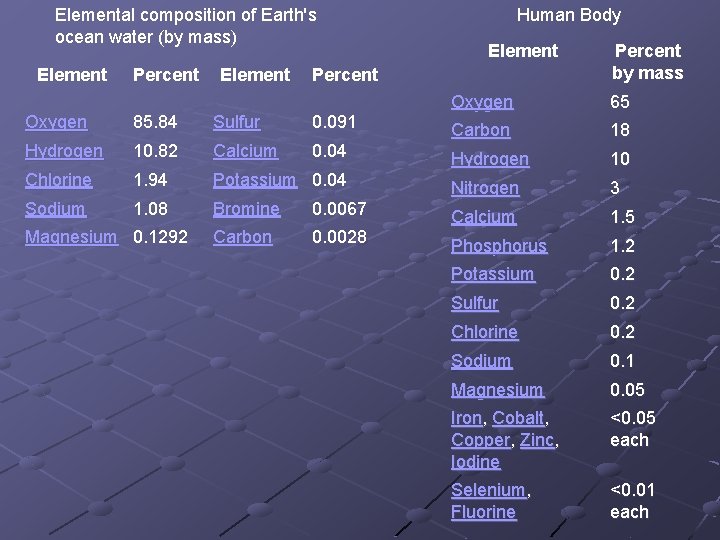
Elemental composition of Earth's ocean water (by mass) Element Percent Oxygen 85. 84 Sulfur 0. 091 Hydrogen 10. 82 Calcium 0. 04 Chlorine 1. 94 Potassium 0. 04 Sodium 1. 08 Bromine 0. 0067 Carbon 0. 0028 Magnesium 0. 1292 Human Body Percent by mass Oxygen 65 Carbon 18 Hydrogen 10 Nitrogen 3 Calcium 1. 5 Phosphorus 1. 2 Potassium 0. 2 Sulfur 0. 2 Chlorine 0. 2 Sodium 0. 1 Magnesium 0. 05 Iron, Cobalt, Copper, Zinc, Iodine <0. 05 each Selenium, Fluorine <0. 01 each



Compounds Substance composed of atoms of two or more different elements that are chemically combined. n n n Ex. water (H 2 O) baking soda (Na. HCO 3) Emerald (Be 3 Al 2(Si. O 3)6)

Why do atoms form chemical bonds? To achieve stability. Electron configuration is the key Full set of electrons in valence shell is most stable arrangement.


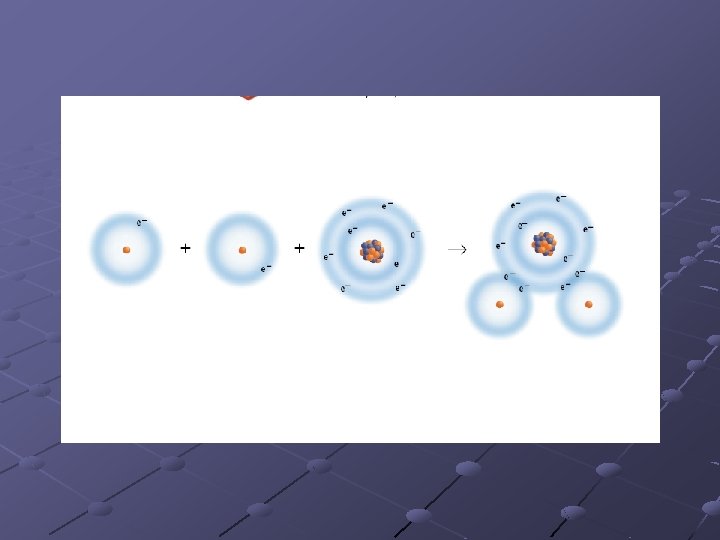
Covalent bonding Bond formed when electrons are shared by atoms. n n Ex. CO 2 Usually forms between two nonmetals

Molecules Composed of two or more atoms held together by covalent bonds. n Have no overall electrical charge Protons equal electrons

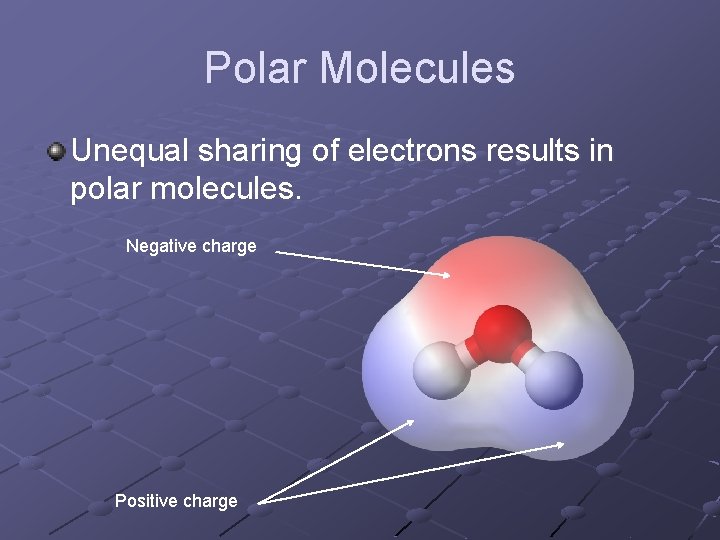
Polar Molecules Unequal sharing of electrons results in polar molecules. Negative charge Positive charge

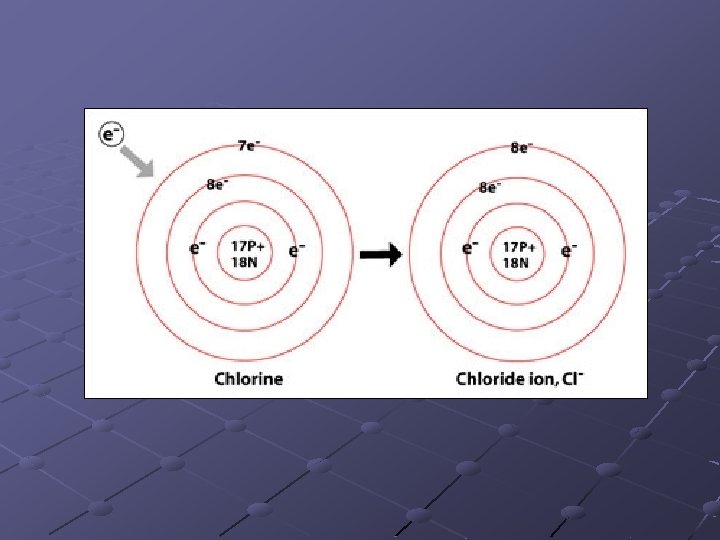
Ions Atom that has gained or lost electrons. Loss or gain of electrons determined by electron stability. Atoms gain or lose electrons as necssary, in order to have a complete (full) valence electron shell

Ionic Bonding that occurs when electrons are donated from one ion to another. Positive ions are always written first in chemical formula. n Ex: Na. Cl where Na+ and Cl-

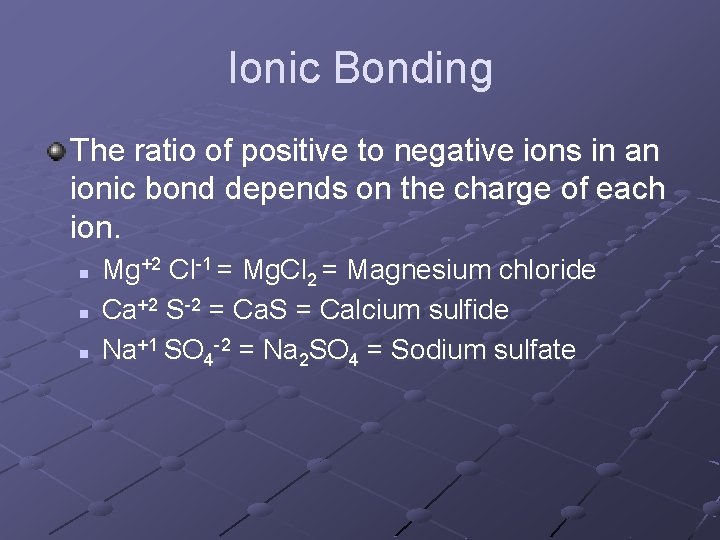
Ionic Bonding The ratio of positive to negative ions in an ionic bond depends on the charge of each ion. n n n Mg+2 Cl-1 = Mg. Cl 2 = Magnesium chloride Ca+2 S-2 = Ca. S = Calcium sulfide Na+1 SO 4 -2 = Na 2 SO 4 = Sodium sulfate

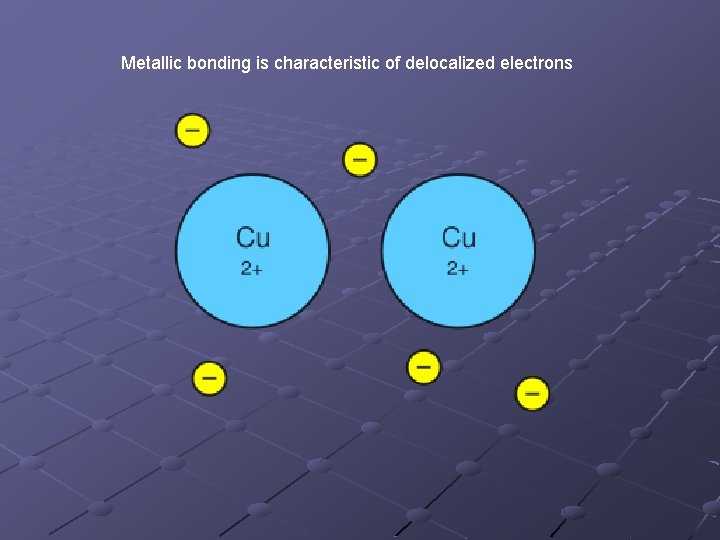
Metallic Bonds Bonding that occurs between atoms of a metal. Accounts for the properties of metals n n n Malleability: ability to be hammered into sheets or shaped Ductility: ability to be pulled or stretched into wire Conductivity: ability to conduct electrical current and transfer thermal energy

Metallic bonding is characteristic of delocalized electrons

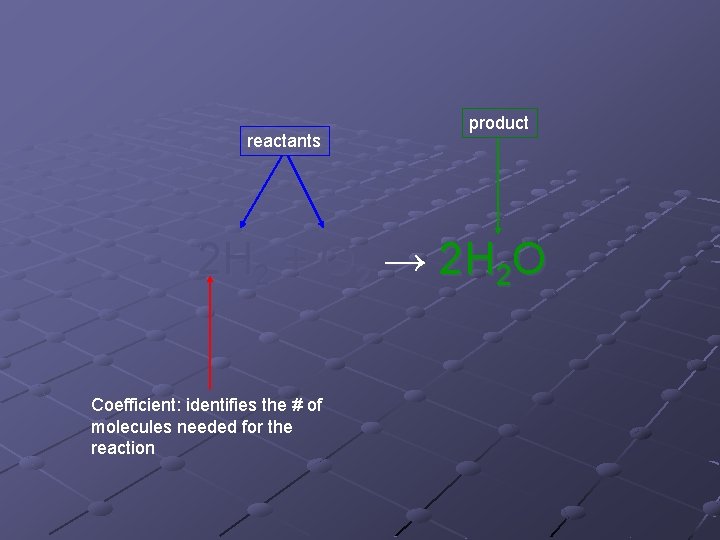
Chemical Reactions Process where substances are changed by chemical means Represented by chemical equations. n n n Identify the reactants and products in a reaction Identify relative numbers of each reactant and product in the reaction Ex. 2 H 2 + O 2 → 2 H 2 O

reactants product 2 H 2 + O 2 → 2 H 2 O Coefficient: identifies the # of molecules needed for the reaction

Mixtures and Solutions Mixtures are combinations of substances that can be separated physically, whereas chemical compounds cannot be separated without chemical processes.

Types of Mixtures Homogeneous: mixture which is uniform (the same) throughout. Ex. Solutions Heterogeneous: mixture which is not uniform throughout (i. e. separate layers)

Acids and Bases An acid is a solution containing a substance the produces Hydrogen ions (H+) in water. n Ex. = HCl, or hydrochloric acid HCl = H+ + Cl-

Acids and Bases A base is a solution containing a substance that produces hydroxide ions (OH-) in water. n Ex. Na. OH, or sodium hydroxide Na. OH = Na+ + OH-

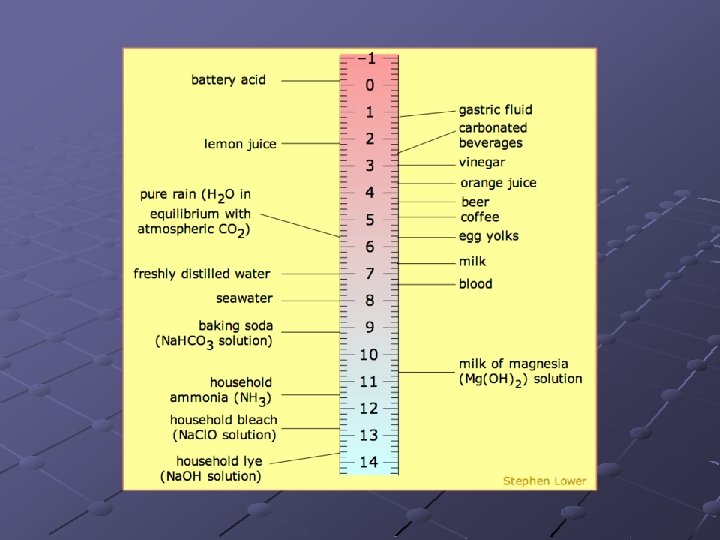
p. H scale Measures the strength of acids and bases relative to each other. p. H identifies the % Hydrogen ions in a solution. Acids and bases can be combined to neutralize each other.
