Matter All matter is composed molecules atoms and


- Slides: 6

Matter • All matter is composed molecules = atoms and groups of atoms bonded together – Pure substances – substances that are made from one type of atom – Compounds – substances that are made from more than one type of atom bonded together – Mixtures – compounds that are combined physically, but not chemically

Elements, Compounds, Mixtures • Element: pure substances, cannot be broken down further • Mixtures: can be separated by physical means – Sand water, shake and let it settle again • Compounds: can only be separated by chemical means – Water – separate the H from the O

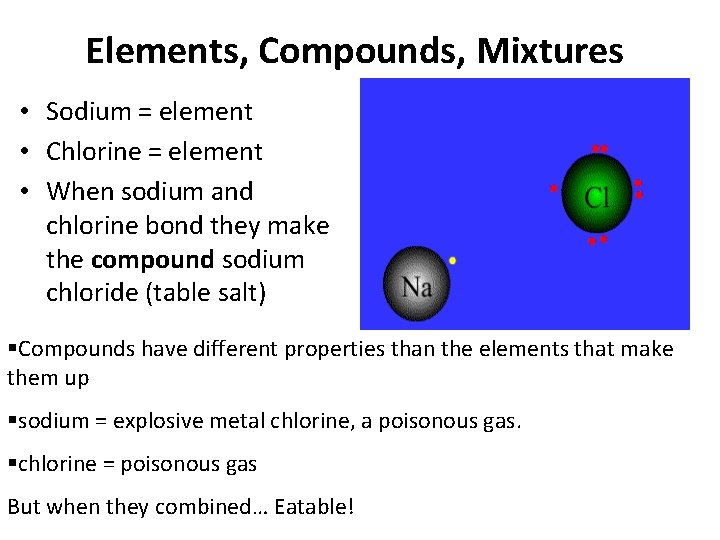
Elements, Compounds, Mixtures • Sodium = element • Chlorine = element • When sodium and chlorine bond they make the compound sodium chloride (table salt) Compounds have different properties than the elements that make them up sodium = explosive metal chlorine, a poisonous gas. chlorine = poisonous gas But when they combined… Eatable!

Elements, Compounds, Mixtures • Hydrogen = element • Oxygen = element • Compound: Hydrogen bonded to oxygen = water • Mixture: Salt combined with water = salt water • Compounds in mixtures retain their individual properties. The ocean is a mixture.

Physical Change vs. Chemical Change • Physical change – the original substance still exists, only the form has changed • Energy changes usually don’t occur • The substance is still the same after a physical change • No change at the molecular level • Chemical change – a new substance is produced • Energy changes always occur • The substance is different after the chemical change • Changes take place of the molecular level

Physical Change vs. Chemical Change • Examples: – Crushing a coke can – Melting an ice cube • Examples: • A nail rusting • Cooking an egg