Mathematics of Chemical Formulas Explain what is Stoichiometry

















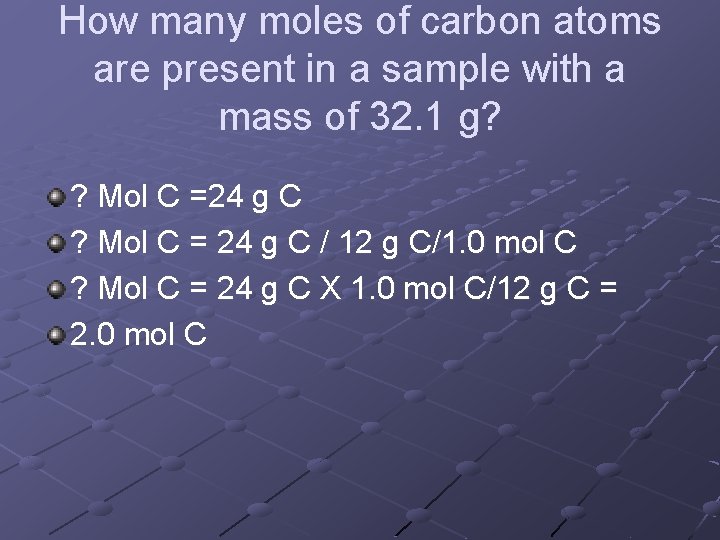



- Slides: 22

Mathematics of Chemical Formulas

Explain what is Stoichiometry

Stoichiometry-Is the study of the quantitative relationships that can be derived from chemical formulas and from chemical equations.

Formula mass- Of a substance is the sum of the atomic masses of all the atoms represented by the formula of the substance. This may be formula of an element, a molecular compound or ionic compound

Molecular Mass-Is the formula mass of a molecular substance

What Units of measurements is more convenient formula mass: 1. amu 2. kilograms 3. grams 4. millimeters.

What is the formula mass of H 2? The subscript “ 2” in the formula means that this formula represents two hydrogen atoms. Because the atomic mass of H is 1. 008 u. You will get the following: 1. 008 + 1. 008 = 2. 016 u

What is the formula mass of H 20? The formula H 2 O represents 3 atoms; 2 H and 1 O atom. The formula mass is the sum of the atomic masses of these 3 atoms. 1. 0 u + 16. 0 u = 18 u

What is the formula mass of calcium hydroxide Element Atomic Mass Atoms/formula = Ca 40 u 1 40 u O 16 u 2 32 u H 1 u 2 2 u ____ Total 74 u

Gram atomic mass -Of an element is that quantity of the element that has a mass in grams numerically equal to its atomic mass. Therefore 16 grams of oxygen is 1 gram atomic mass of oxygen AKA gram-atom

Gram formula mass is the quantity of a substance that has a mass in grams numerically equal to its formula mass. Since the formula mass of water is 18 u the gram formula mass of water is 18 grams

Gram molecular mass = gram formula mass in working with molecules

Mole-aka mol is the number of atoms in 1 gram atomic mass of an element. This number is 6. 02 X 1023. This can refer to 6. 02 X 1023 of anything

Mole Relationships N atoms (6. 02 X 1023 atoms) in 1 gram atomic mass of an element. There are N molecules (6. 02 X 1023 ) molecules in 1 gram molecular mass of every molecular substance N formula units in 1 gram formula mass of any substance

Mole Relationships Aluminum 1 mole 1 gram atomic mass 27 grams 6. 02 X 1023 atoms

Mole relationships Water 1 mole 1 gram formula mass 18 grams 6. 02 X 1023 molecules

What is the mass in grams of 1. 00 mole of sulfur atoms? One mole of sulfur atoms is the number of atoms in the gram atomic mass of sulfur. Amu =32. 1 u The gram atomic mass of sulfur is 32. 1 g Therefore the mass of 1. 00 mole of sulfur atoms is 32. 1 g

What is the mass of 5. 0 moles of sulfur? 5. 0 moles S atoms X 32. 1 g S 1. 00 moles of S atoms = 160 g S or 1. 6 X 102 g S
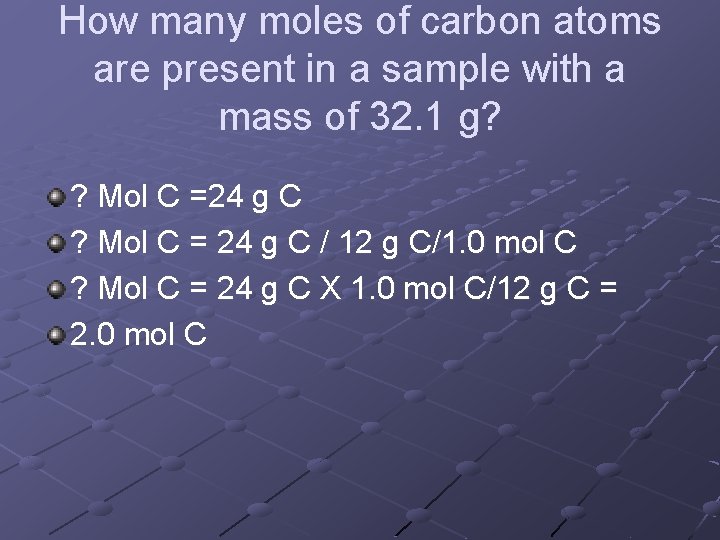
How many moles of carbon atoms are present in a sample with a mass of 32. 1 g? ? Mol C =24 g C ? Mol C = 24 g C / 12 g C/1. 0 mol C ? Mol C = 24 g C X 1. 0 mol C/12 g C = 2. 0 mol C

How many carbon atoms would there be in the sample? ? Atoms C = 2. 0 mol C X 6. 02 X 1023 atoms C / mol C ? Atoms C = 12. 04 X 1023 atoms C or 1. 2 X 1024 atoms C

Other concepts will be forthcoming later
