MATHEMATICS OF CHEMICAL FORMULAS CHEMISTRY CHAPTER 6 6


- Slides: 18

MATHEMATICS OF CHEMICAL FORMULAS CHEMISTRY CHAPTER 6

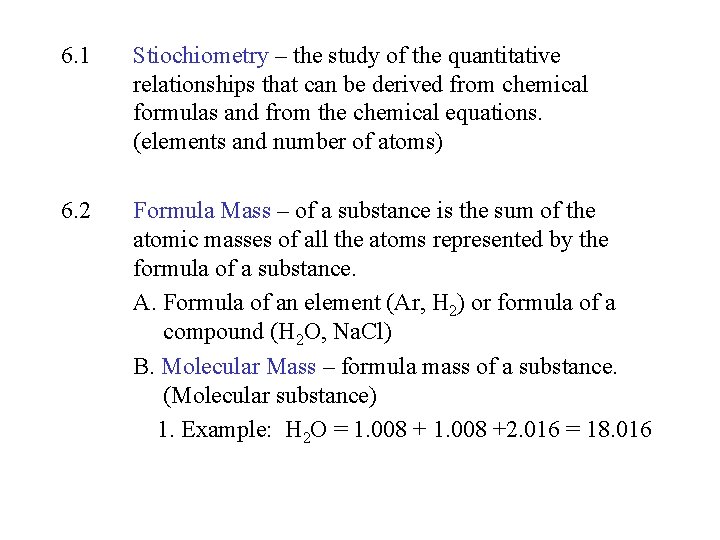
6. 1 Stiochiometry – the study of the quantitative relationships that can be derived from chemical formulas and from the chemical equations. (elements and number of atoms) 6. 2 Formula Mass – of a substance is the sum of the atomic masses of all the atoms represented by the formula of a substance. A. Formula of an element (Ar, H 2) or formula of a compound (H 2 O, Na. Cl) B. Molecular Mass – formula mass of a substance. (Molecular substance) 1. Example: H 2 O = 1. 008 +2. 016 = 18. 016

6. 3 Gram Atomic Mass and Gram Formula Mass A. Gram Atomic Mass – of an element is the quantity of the element that has a mass numerically equal to its atomic number. 1. Example: atomic mass of oxygen is 16 u, which is equal to 16 grams of oxygen, which is 1 gram atomic mass of oxygen. (Called a gram atom) B. Gram Formula Mass – is the quantity of a substance that has a mass in grams numerically equal to its formula mass. 1. Example: Water has a formula mass of 18 u, the gram formula mass is 18 gram.

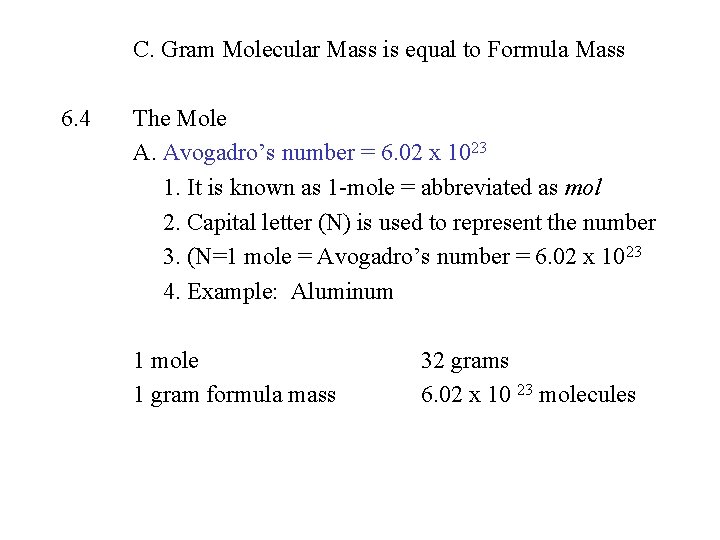
C. Gram Molecular Mass is equal to Formula Mass 6. 4 The Mole A. Avogadro’s number = 6. 02 x 1023 1. It is known as 1 -mole = abbreviated as mol 2. Capital letter (N) is used to represent the number 3. (N=1 mole = Avogadro’s number = 6. 02 x 1023 4. Example: Aluminum 1 mole 1 gram formula mass 32 grams 6. 02 x 10 23 molecules

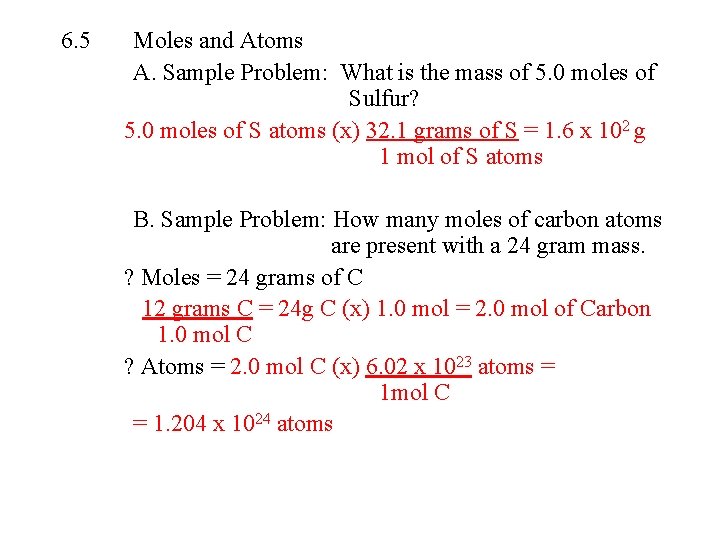
6. 5 Moles and Atoms A. Sample Problem: What is the mass of 5. 0 moles of Sulfur? 5. 0 moles of S atoms (x) 32. 1 grams of S = 1. 6 x 102 g 1 mol of S atoms B. Sample Problem: How many moles of carbon atoms are present with a 24 gram mass. ? Moles = 24 grams of C 12 grams C = 24 g C (x) 1. 0 mol = 2. 0 mol of Carbon 1. 0 mol C ? Atoms = 2. 0 mol C (x) 6. 02 x 1023 atoms = 1 mol C = 1. 204 x 1024 atoms

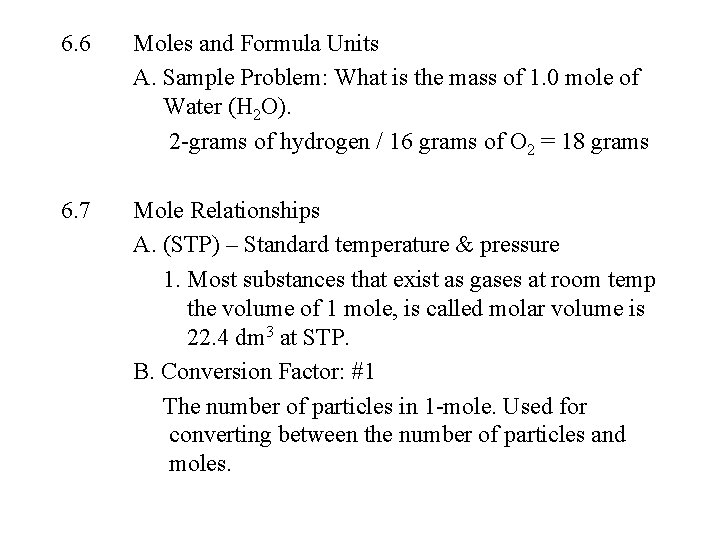
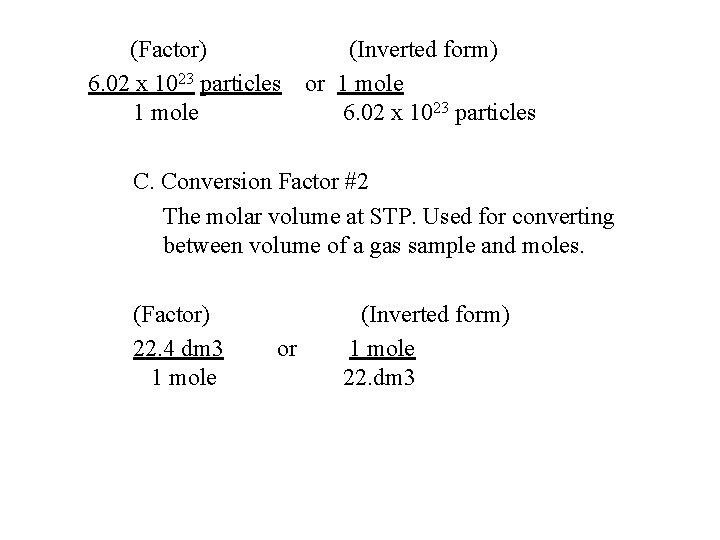
6. 6 Moles and Formula Units A. Sample Problem: What is the mass of 1. 0 mole of Water (H 2 O). 2 -grams of hydrogen / 16 grams of O 2 = 18 grams 6. 7 Mole Relationships A. (STP) – Standard temperature & pressure 1. Most substances that exist as gases at room temp the volume of 1 mole, is called molar volume is 22. 4 dm 3 at STP. B. Conversion Factor: #1 The number of particles in 1 -mole. Used for converting between the number of particles and moles.

(Factor) 6. 02 x 1023 particles 1 mole (Inverted form) or 1 mole 6. 02 x 1023 particles C. Conversion Factor #2 The molar volume at STP. Used for converting between volume of a gas sample and moles. (Factor) 22. 4 dm 3 1 mole or (Inverted form) 1 mole 22. dm 3

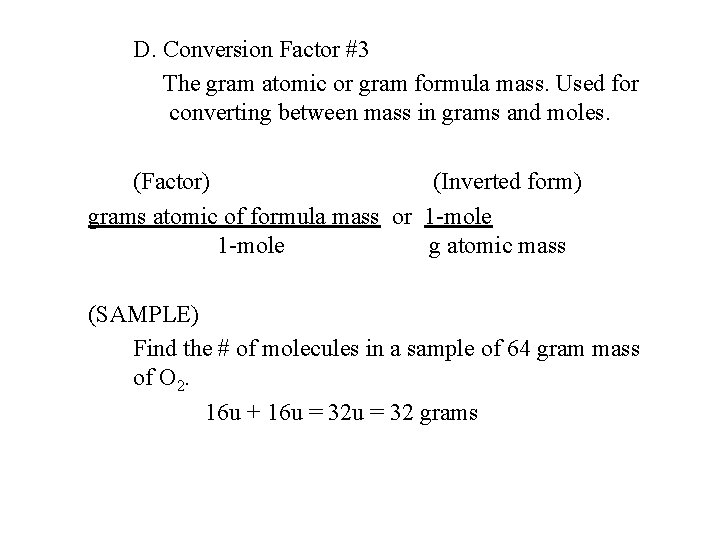
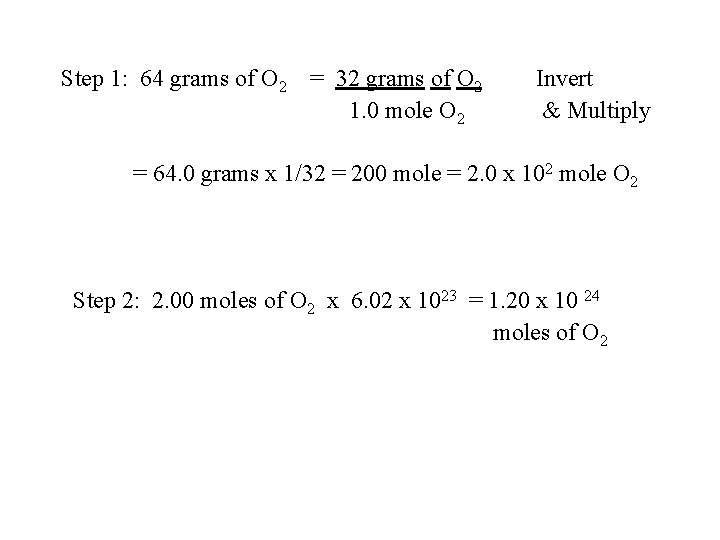
D. Conversion Factor #3 The gram atomic or gram formula mass. Used for converting between mass in grams and moles. (Factor) (Inverted form) grams atomic of formula mass or 1 -mole g atomic mass (SAMPLE) Find the # of molecules in a sample of 64 gram mass of O 2. 16 u + 16 u = 32 grams

Step 1: 64 grams of O 2 = 32 grams of O 2 1. 0 mole O 2 Invert & Multiply = 64. 0 grams x 1/32 = 200 mole = 2. 0 x 102 mole O 2 Step 2: 2. 00 moles of O 2 x 6. 02 x 1023 = 1. 20 x 10 24 moles of O 2

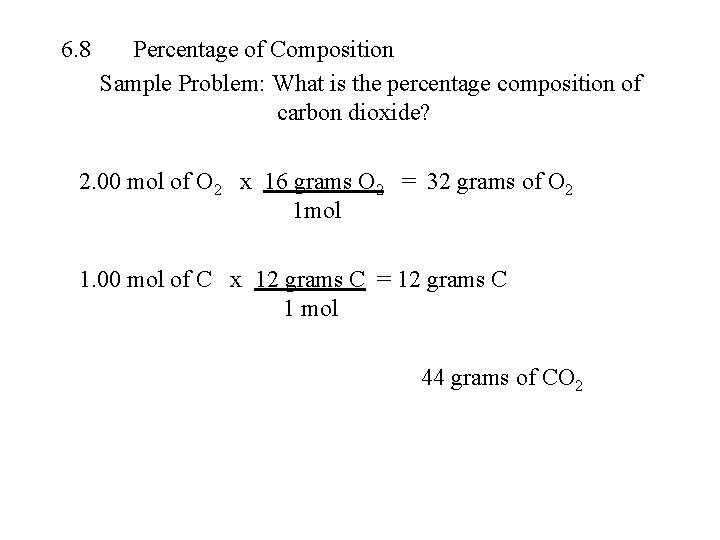
6. 8 Percentage of Composition Sample Problem: What is the percentage composition of carbon dioxide? 2. 00 mol of O 2 x 16 grams O 2 = 32 grams of O 2 1 mol 1. 00 mol of C x 12 grams C = 12 grams C 1 mol 44 grams of CO 2

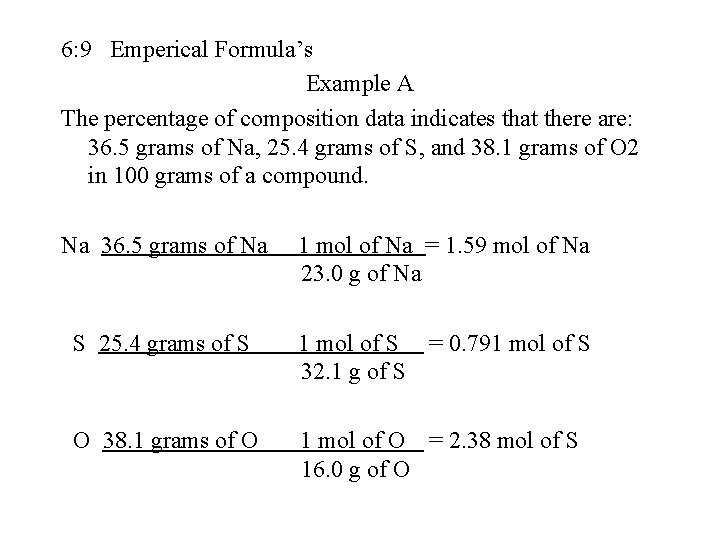
6: 9 Emperical Formula’s Example A The percentage of composition data indicates that there are: 36. 5 grams of Na, 25. 4 grams of S, and 38. 1 grams of O 2 in 100 grams of a compound. Na 36. 5 grams of Na 1 mol of Na = 1. 59 mol of Na 23. 0 g of Na S 25. 4 grams of S 1 mol of S = 0. 791 mol of S 32. 1 g of S O 38. 1 grams of O 1 mol of O = 2. 38 mol of S 16. 0 g of O

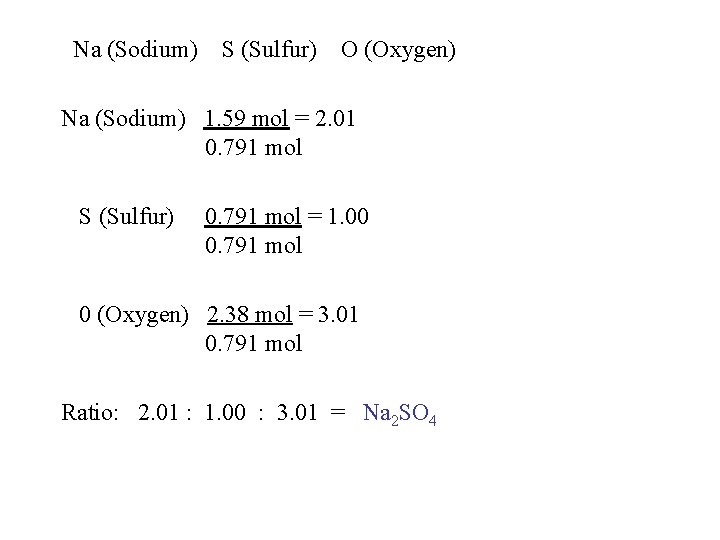
Na (Sodium) S (Sulfur) O (Oxygen) Na (Sodium) 1. 59 mol = 2. 01 0. 791 mol S (Sulfur) 0. 791 mol = 1. 00 0. 791 mol 0 (Oxygen) 2. 38 mol = 3. 01 0. 791 mol Ratio: 2. 01 : 1. 00 : 3. 01 = Na 2 SO 4

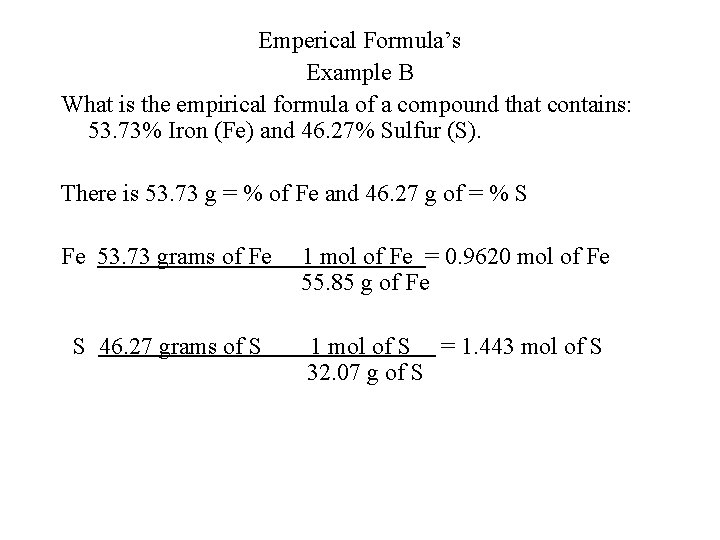
Emperical Formula’s Example B What is the empirical formula of a compound that contains: 53. 73% Iron (Fe) and 46. 27% Sulfur (S). There is 53. 73 g = % of Fe and 46. 27 g of = % S Fe 53. 73 grams of Fe 1 mol of Fe = 0. 9620 mol of Fe 55. 85 g of Fe S 46. 27 grams of S 1 mol of S = 1. 443 mol of S 32. 07 g of S

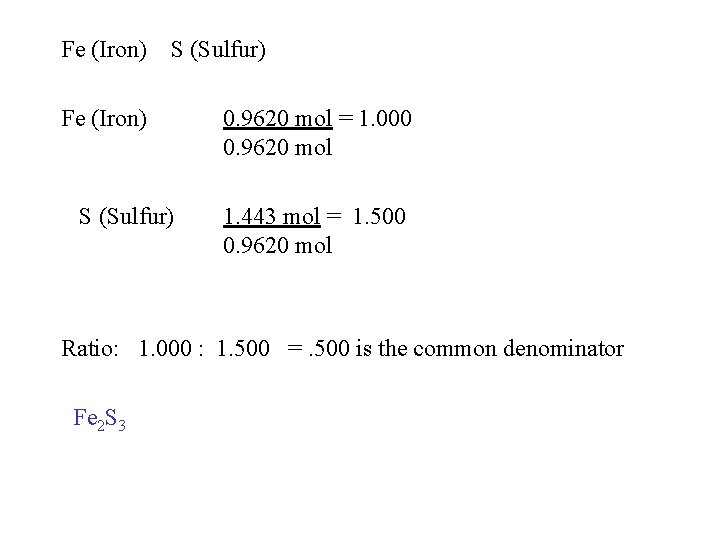
Fe (Iron) S (Sulfur) 0. 9620 mol = 1. 000 0. 9620 mol 1. 443 mol = 1. 500 0. 9620 mol Ratio: 1. 000 : 1. 500 =. 500 is the common denominator Fe 2 S 3

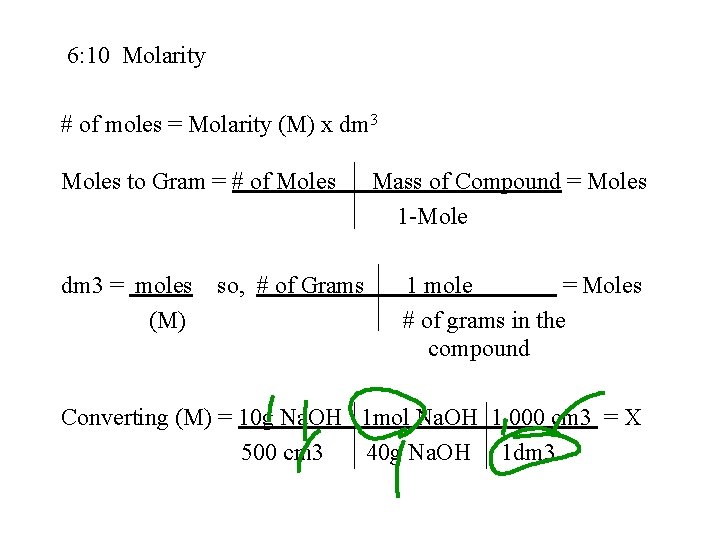
6: 10 Molarity # of moles = Molarity (M) x dm 3 Moles to Gram = # of Moles dm 3 = moles (M) so, # of Grams Mass of Compound = Moles 1 -Mole 1 mole = Moles # of grams in the compound Converting (M) = 10 g Na. OH 1 mol Na. OH 1. 000 cm 3 = X 500 cm 3 40 g Na. OH 1 dm 3

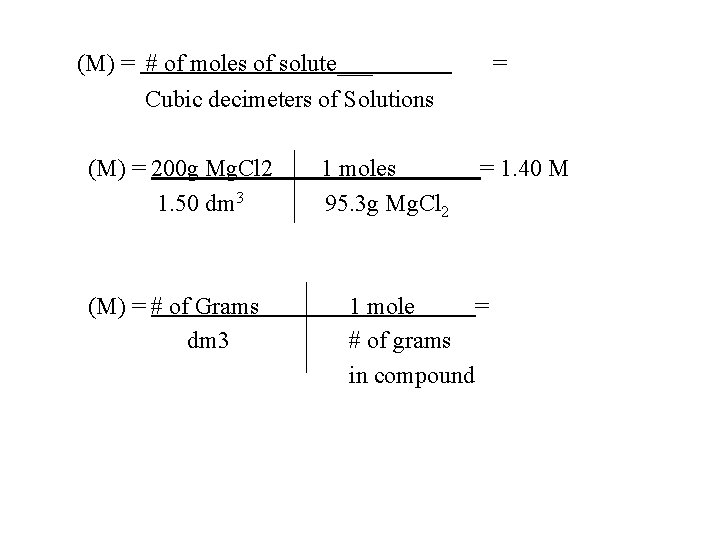
(M) = # of moles of solute___ = Cubic decimeters of Solutions (M) = 200 g Mg. Cl 2 1. 50 dm 3 (M) = # of Grams dm 3 1 moles 95. 3 g Mg. Cl 2 = 1. 40 M 1 mole = # of grams in compound

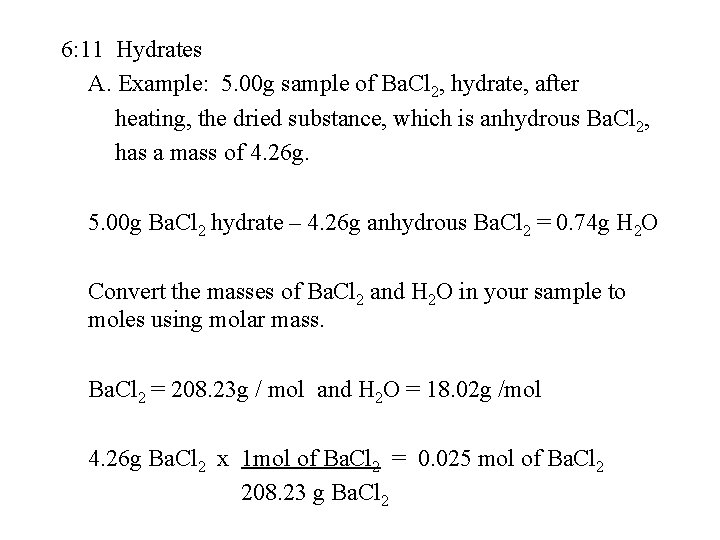
6: 11 Hydrates A. Example: 5. 00 g sample of Ba. Cl 2, hydrate, after heating, the dried substance, which is anhydrous Ba. Cl 2, has a mass of 4. 26 g. 5. 00 g Ba. Cl 2 hydrate – 4. 26 g anhydrous Ba. Cl 2 = 0. 74 g H 2 O Convert the masses of Ba. Cl 2 and H 2 O in your sample to moles using molar mass. Ba. Cl 2 = 208. 23 g / mol and H 2 O = 18. 02 g /mol 4. 26 g Ba. Cl 2 x 1 mol of Ba. Cl 2 = 0. 025 mol of Ba. Cl 2 208. 23 g Ba. Cl 2

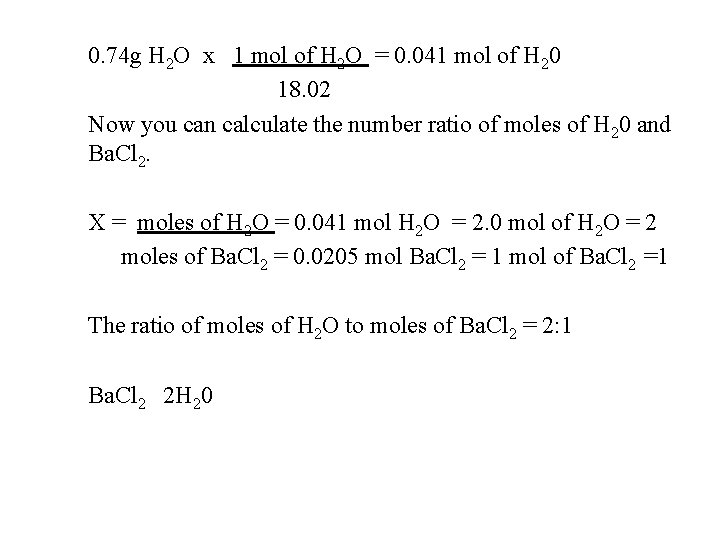
0. 74 g H 2 O x 1 mol of H 2 O = 0. 041 mol of H 20 18. 02 Now you can calculate the number ratio of moles of H 20 and Ba. Cl 2. X = moles of H 2 O = 0. 041 mol H 2 O = 2. 0 mol of H 2 O = 2 moles of Ba. Cl 2 = 0. 0205 mol Ba. Cl 2 = 1 mol of Ba. Cl 2 =1 The ratio of moles of H 2 O to moles of Ba. Cl 2 = 2: 1 Ba. Cl 2 2 H 20