Math Skill Test Study Guide Math Test Questions






- Slides: 22

Math Skill Test Study Guide

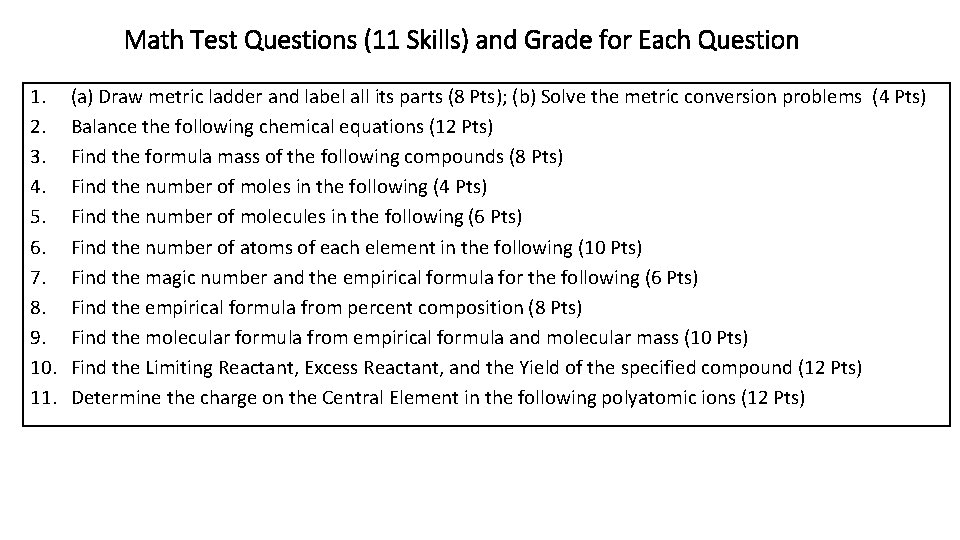
Math Test Questions (11 Skills) and Grade for Each Question 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. (a) Draw metric ladder and label all its parts (8 Pts); (b) Solve the metric conversion problems (4 Pts) Balance the following chemical equations (12 Pts) Find the formula mass of the following compounds (8 Pts) Find the number of moles in the following (4 Pts) Find the number of molecules in the following (6 Pts) Find the number of atoms of each element in the following (10 Pts) Find the magic number and the empirical formula for the following (6 Pts) Find the empirical formula from percent composition (8 Pts) Find the molecular formula from empirical formula and molecular mass (10 Pts) Find the Limiting Reactant, Excess Reactant, and the Yield of the specified compound (12 Pts) Determine the charge on the Central Element in the following polyatomic ions (12 Pts)

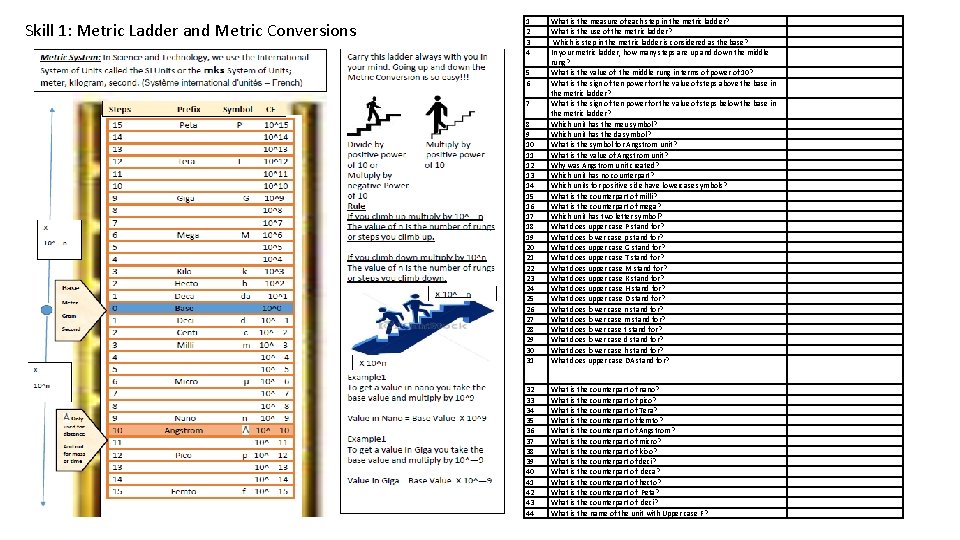
Skill 1: Metric Ladder and Metric Conversions 1 2 3 4 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 What is the measure of each step in the metric ladder? What is the use of the metric ladder? Which is step in the metric ladder is considered as the base? In your metric ladder, how many steps are up and down the middle rung? What is the value of the middle rung in terms of power of 10? What is the sign of ten power for the value of steps above the base in the metric ladder? What is the sign of ten power for the value of steps below the base in the metric ladder? Which unit has the meu symbol? Which unit has the da symbol? What is the symbol for Angstrom unit? What is the value of Angstrom unit? Why was Angstrom unit created? Which unit has no counterpart? Which units for positive side have lowercase symbols? What is the counterpart of milli? What is the counterpart of mega? Which unit has two letter symbol? What does upper case P stand for? What does lower case p stand for? What does upper case G stand for? What does upper case T stand for? What does upper case M stand for? What does upper case K stand for? What does upper case H stand for? What does upper case D stand for? What does lower case n stand for? What does lower case m stand for? What does lower case t stand for? What does lower case d stand for? What does lower case h stand for? What does upper case DA stand for? 32 33 34 35 36 37 38 39 40 41 42 43 44 What is the counterpart of nano? What is the counterpart of pico? What is the counterpart of Tera? What is the counterpart of femto? What is the counterpart of Angstrom? What is the counterpart of micro? What is the counterpart of kioo? What is the counterpart of deci? What is the counterpart of deca? What is the counterpart of hecto? What is the counterpart of Peta? What is the counterpart of deci? What is the name of the unit with Upper case F? 5 6 7

Your Turn: Skill 1 • Draw and practice metric ladder • Practice all Metric conversions in the given chart

Skill 2: Balancing Chemical Equations Watch video at https: //www. google. com/search? q=steps+to+balanci ng+chemical+equations&oq=steps+to+balancing+che mical+equations&aqs=chrome. . 69 i 57 j 0 l 5. 9232 j 0 j 7&so urceid=chrome&ie=UTF-8#kpvalbx=1 Try problems given on the left Take Practice test at http: //www. sciencegeek. net/Chemistry/Review/Balan cing/ Further do a plenty of problems given at https: //www. northallegheny. org/cms/lib/PA 0100111 9/Centricity/Domain/1083/balancingpractice. pdf

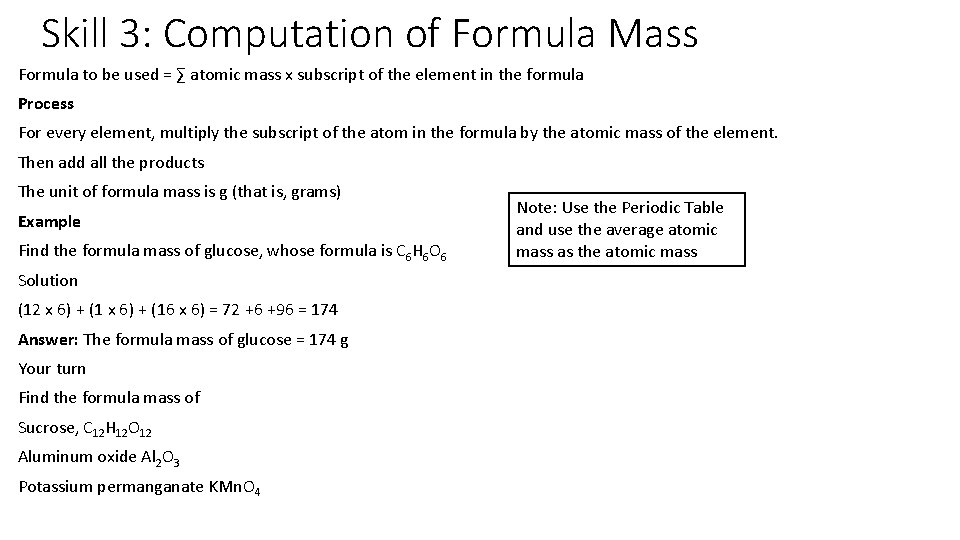
Skill 3: Computation of Formula Mass Formula to be used = ∑ atomic mass x subscript of the element in the formula Process For every element, multiply the subscript of the atom in the formula by the atomic mass of the element. Then add all the products The unit of formula mass is g (that is, grams) Example Find the formula mass of glucose, whose formula is C 6 H 6 O 6 Solution (12 x 6) + (16 x 6) = 72 +6 +96 = 174 Answer: The formula mass of glucose = 174 g Your turn Find the formula mass of Sucrose, C 12 H 12 O 12 Aluminum oxide Al 2 O 3 Potassium permanganate KMn. O 4 Note: Use the Periodic Table and use the average atomic mass as the atomic mass

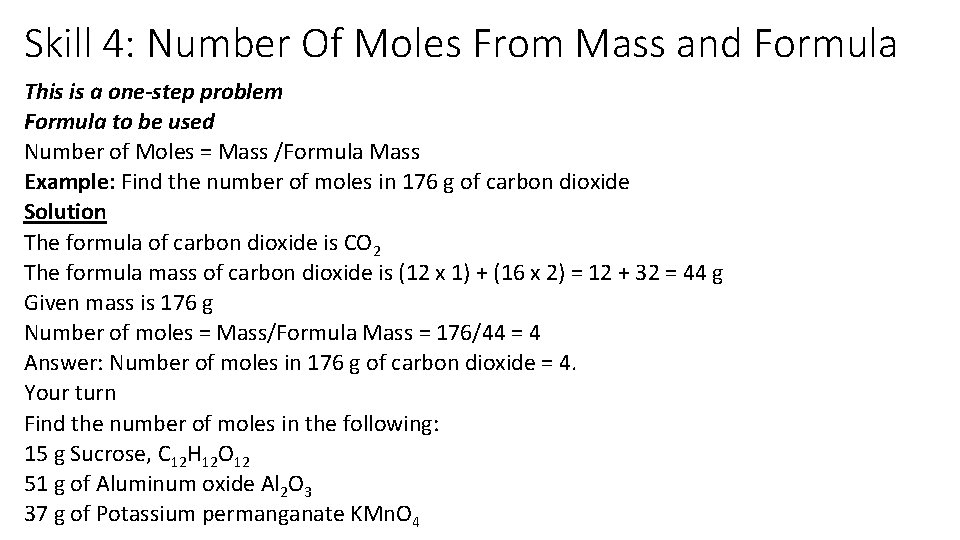
Skill 4: Number Of Moles From Mass and Formula This is a one-step problem Formula to be used Number of Moles = Mass /Formula Mass Example: Find the number of moles in 176 g of carbon dioxide Solution The formula of carbon dioxide is CO 2 The formula mass of carbon dioxide is (12 x 1) + (16 x 2) = 12 + 32 = 44 g Given mass is 176 g Number of moles = Mass/Formula Mass = 176/44 = 4 Answer: Number of moles in 176 g of carbon dioxide = 4. Your turn Find the number of moles in the following: 15 g Sucrose, C 12 H 12 O 12 51 g of Aluminum oxide Al 2 O 3 37 g of Potassium permanganate KMn. O 4

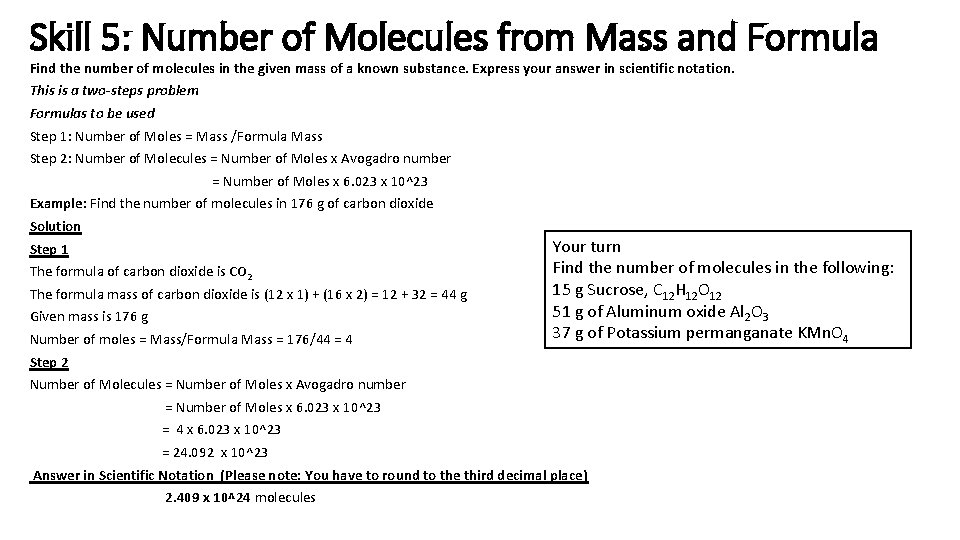
Skill 5: Number of Molecules from Mass and Formula Find the number of molecules in the given mass of a known substance. Express your answer in scientific notation. This is a two-steps problem Formulas to be used Step 1: Number of Moles = Mass /Formula Mass Step 2: Number of Molecules = Number of Moles x Avogadro number = Number of Moles x 6. 023 x 10^23 Example: Find the number of molecules in 176 g of carbon dioxide Solution Step 1 The formula of carbon dioxide is CO 2 The formula mass of carbon dioxide is (12 x 1) + (16 x 2) = 12 + 32 = 44 g Given mass is 176 g Number of moles = Mass/Formula Mass = 176/44 = 4 Your turn Find the number of molecules in the following: 15 g Sucrose, C 12 H 12 O 12 51 g of Aluminum oxide Al 2 O 3 37 g of Potassium permanganate KMn. O 4 Step 2 Number of Molecules = Number of Moles x Avogadro number = Number of Moles x 6. 023 x 10^23 = 4 x 6. 023 x 10^23 = 24. 092 x 10^23 Answer in Scientific Notation (Please note: You have to round to the third decimal place) 2. 409 x 10^24 molecules

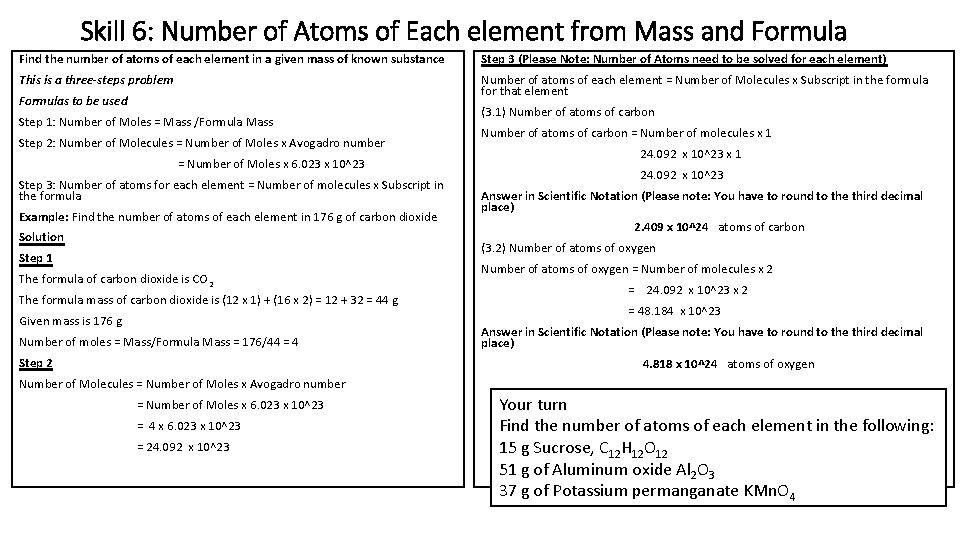
Skill 6: Number of Atoms of Each element from Mass and Formula Find the number of atoms of each element in a given mass of known substance Step 3 (Please Note: Number of Atoms need to be solved for each element) This is a three-steps problem Number of atoms of each element = Number of Molecules x Subscript in the formula for that element Formulas to be used Step 1: Number of Moles = Mass /Formula Mass Step 2: Number of Molecules = Number of Moles x Avogadro number = Number of Moles x 6. 023 x 10^23 Step 3: Number of atoms for each element = Number of molecules x Subscript in the formula Example: Find the number of atoms of each element in 176 g of carbon dioxide Solution Step 1 The formula of carbon dioxide is CO 2 The formula mass of carbon dioxide is (12 x 1) + (16 x 2) = 12 + 32 = 44 g Given mass is 176 g (3. 1) Number of atoms of carbon = Number of molecules x 1 24. 092 x 10^23 Answer in Scientific Notation (Please note: You have to round to the third decimal place) 2. 409 x 10^24 atoms of carbon (3. 2) Number of atoms of oxygen = Number of molecules x 2 = 24. 092 x 10^23 x 2 = 48. 184 x 10^23 Number of moles = Mass/Formula Mass = 176/44 = 4 Answer in Scientific Notation (Please note: You have to round to the third decimal place) Step 2 4. 818 x 10^24 atoms of oxygen Number of Molecules = Number of Moles x Avogadro number = Number of Moles x 6. 023 x 10^23 = 4 x 6. 023 x 10^23 = 24. 092 x 10^23 Your turn Find the number of atoms of each element in the following: 15 g Sucrose, C 12 H 12 O 12 51 g of Aluminum oxide Al 2 O 3 37 g of Potassium permanganate KMn. O 4

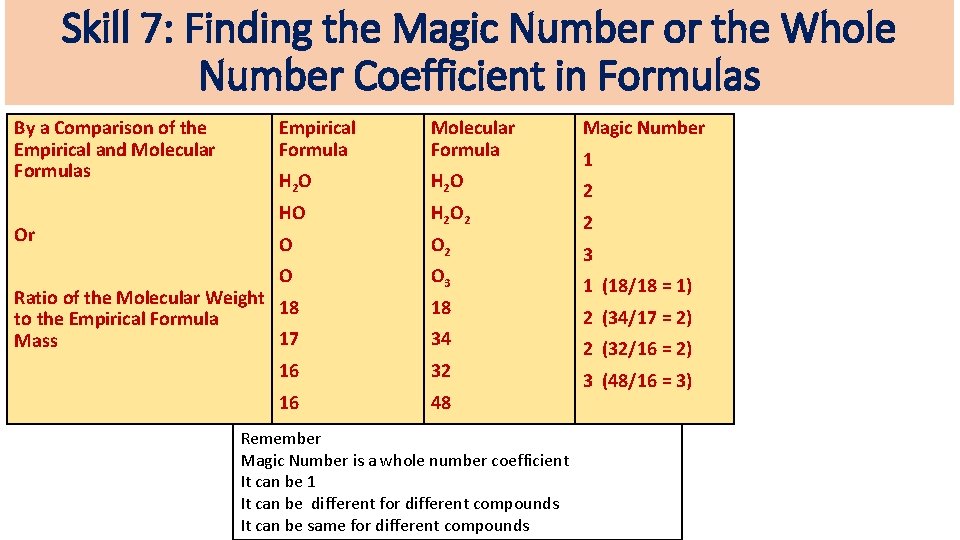
Skill 7: Finding the Magic Number or the Whole Number Coefficient in Formulas By a Comparison of the Empirical and Molecular Formulas Or Empirical Formula Molecular Formula Magic Number H 2 O HO H 2 O 2 2 O O 3 Ratio of the Molecular Weight 18 to the Empirical Formula 17 Mass 16 16 18 34 32 48 Remember Magic Number is a whole number coefficient It can be 1 It can be different for different compounds It can be same for different compounds 1 2 3 1 (18/18 = 1) 2 (34/17 = 2) 2 (32/16 = 2) 3 (48/16 = 3)

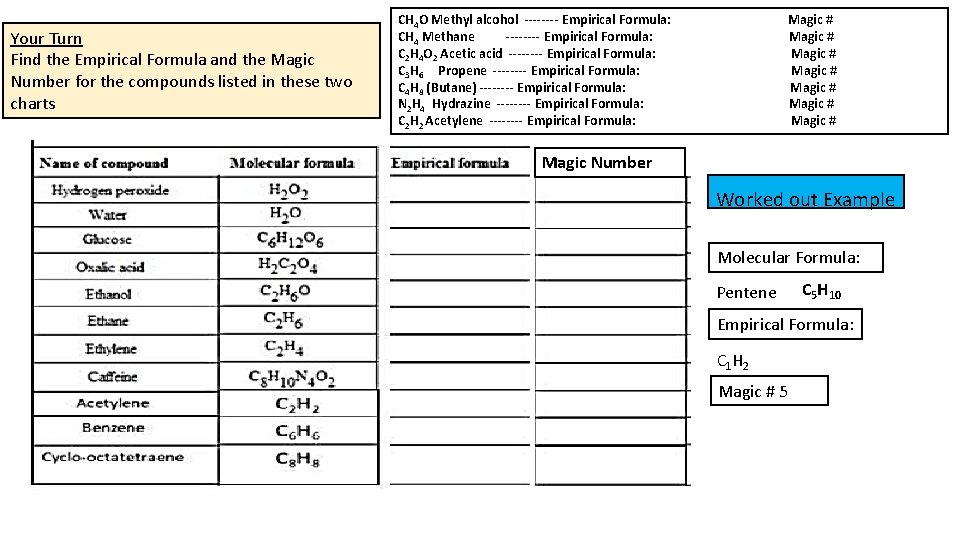
Your Turn Find the Empirical Formula and the Magic Number for the compounds listed in these two charts CH 4 O Methyl alcohol ---- Empirical Formula: Magic # CH 4 Methane ---- Empirical Formula: Magic # C 2 H 4 O 2 Acetic acid ---- Empirical Formula: Magic # C 3 H 6 Propene ---- Empirical Formula: Magic # C 4 H 8 (Butane) ---- Empirical Formula: Magic # N 2 H 4 Hydrazine ---- Empirical Formula: Magic # C 2 H 2 Acetylene ---- Empirical Formula: Magic # Magic Number Worked out Example Molecular Formula: Pentene C 5 H 10 Empirical Formula: C 1 H 2 Magic # 5

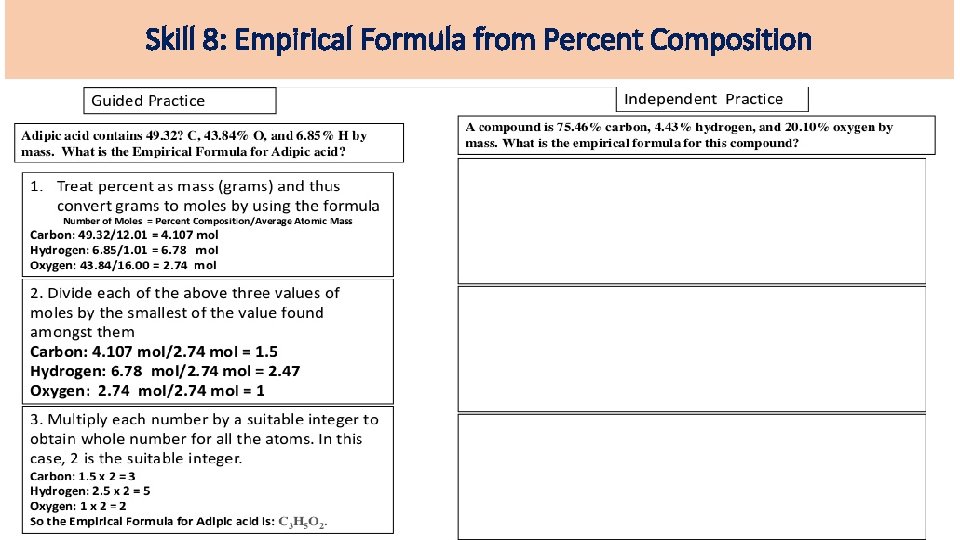
Skill 8: Empirical Formula from Percent Composition

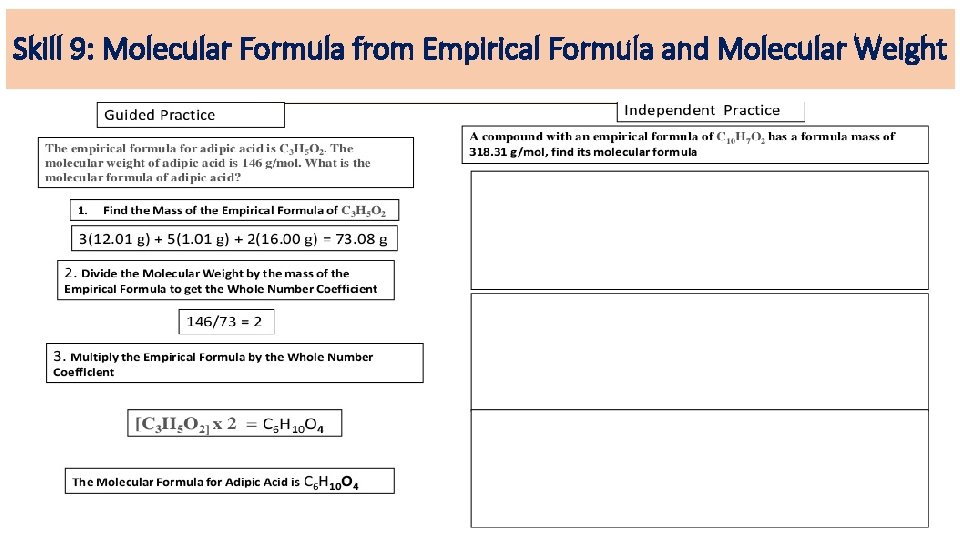
Skill 9: Molecular Formula from Empirical Formula and Molecular Weight

Skill 10: Law of Conservation of Mass A thin strip of iron with a mass of 15. 5 g is placed into a solution containing 21. 0 g of copper (II) sulfate and copper begins to form. After some times, the reactions stops because all the copper (II) sulfate has reacted. The iron strip is found to have a mass of 8. 5 g. The mass of copper formed is found to be 8. 60 g. What mass of iron (II) sulfate has been formed in the reaction? Solution: Mass of iron = Initial mass – Final mass = 15. 5 – 8. 5 = 7. 0 g Mass of copper (II) sulfate = 21. 0 g Mass of copper = 8. 60 g According to law of conservation of mass, Mass of iron + Mass of copper (II) sulfate = Mass of copper + Mass of iron (II) sulfate 7. 0 g + 21. 0 g = 8. 60 g + Mass of iron (II) sulfate So, Mass of iron (II) sulfate = 19. 40 g Your Turn When 33 g of wood was burned in air, it combined with 32 g of oxygen to form 3 g of ash and a gas mixture of carbon dioxide and water. When the gas mixture was cooled, 18 g of water condensed. How much carbon dioxide was formed? A student adds 15 g of baking soda to 10 g of acetic acid in a beaker. A chemical reaction occurs and a gas is given off. After the reaction, the mass of the products remaining in the beaker is 23 g. Has mass been conserved in this reaction? Explain. ___________________________________________________________ ______________________________ Is this system open or closed? ___________________________________________________________

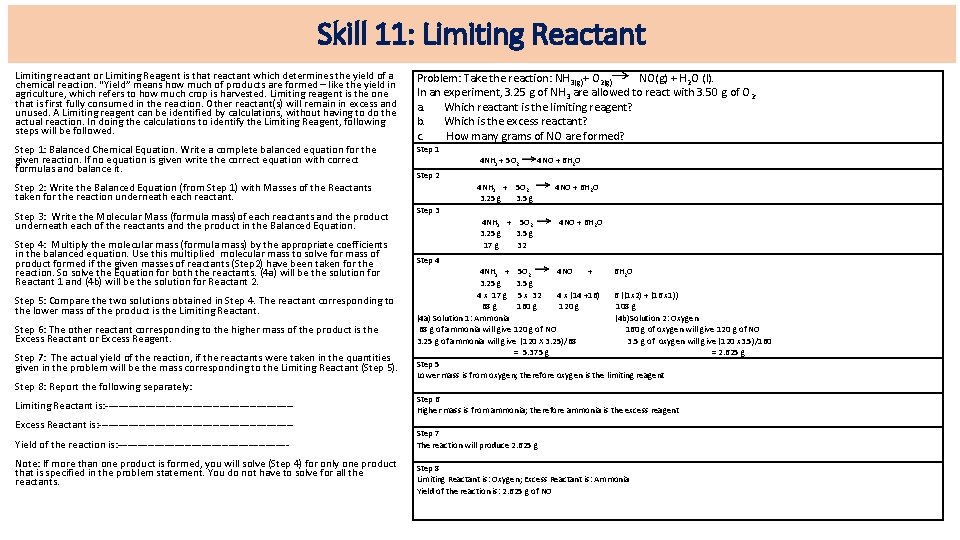
Skill 11: Limiting Reactant Limiting reactant or Limiting Reagent is that reactant which determines the yield of a chemical reaction. “Yield” means how much of products are formed – like the yield in agriculture, which refers to how much crop is harvested. Limiting reagent is the one that is first fully consumed in the reaction. Other reactant(s) will remain in excess and unused. A Limiting reagent can be identified by calculations, without having to do the actual reaction. In doing the calculations to identify the Limiting Reagent, following steps will be followed. Problem: Take the reaction: NH 3(g)+ O 2(g) NO(g) + H 2 O (l). In an experiment, 3. 25 g of NH 3 are allowed to react with 3. 50 g of O 2. a. Which reactant is the limiting reagent? b. Which is the excess reactant? c. How many grams of NO are formed? Step 1: Balanced Chemical Equation. Write a complete balanced equation for the given reaction. If no equation is given write the correct equation with correct formulas and balance it. Step 1 4 NH 3 + 5 O 2 4 NO + 6 H 2 O Step 2: Write the Balanced Equation (from Step 1) with Masses of the Reactants taken for the reaction underneath each reactant. Step 3: Write the Molecular Mass (formula mass)of each reactants and the product underneath each of the reactants and the product in the Balanced Equation. Step 4: Multiply the molecular mass (formula mass) by the appropriate coefficients in the balanced equation. Use this multiplied molecular mass to solve for mass of product formed if the given masses of reactants (Step 2) have been taken for the reaction. So solve the Equation for both the reactants. (4 a) will be the solution for Reactant 1 and (4 b) will be the solution for Reactant 2. Step 5: Compare the two solutions obtained in Step 4. The reactant corresponding to the lower mass of the product is the Limiting Reactant. Step 6: The other reactant corresponding to the higher mass of the product is the Excess Reactant or Excess Reagent. Step 7: The actual yield of the reaction, if the reactants were taken in the quantities given in the problem will be the mass corresponding to the Limiting Reactant (Step 5). Step 8: Report the following separately: Limiting Reactant is: ----------------------------Excess Reactant is: -----------------------------Yield of the reaction is: -------------------------- Note: If more than one product is formed, you will solve (Step 4) for only one product that is specified in the problem statement. You do not have to solve for all the reactants. Step 2 4 NH 3 + 5 O 2 4 NO + 6 H 2 O 3. 25 g 3. 5 g Step 3 4 NH 3 + 5 O 2 4 NO + 6 H 2 O 3. 25 g 3. 5 g 17 g 32 Step 4 4 NH 3 + 5 O 2 4 NO + 6 H 2 O 3. 25 g 3. 5 g 4 x 17 g 5 x 32 4 x (14 +16) 6 ((1 x 2) + (16 x 1)) 68 g 160 g 120 g 108 g (4 a) Solution 1: Ammonia (4 b)Solution 2: Oxygen 68 g of ammonia will give 120 g of NO 160 g of oxygen will give 120 g of NO 3. 25 g of ammonia will give (120 X 3. 25)/68 3. 5 g of oxygen will give (120 x 3. 5)/160 = 5. 375 g = 2. 625 g Step 5 Lower mass is from oxygen; therefore oxygen is the limiting reagent Step 6 Higher mass is from ammonia; therefore ammonia is the excess reagent Step 7 The reaction will produce 2. 625 g Step 8 Limiting Reactant is: Oxygen; Excess Reactant is: Ammonia Yield of the reaction is: 2. 625 g of NO

Your Turn: Practice Problem 1: Skill 8: Limiting Reactant Problem 1: Take the reaction: Al + O 2 Al 2 O 3 In an experiment, 54 g of aluminum is allowed to react with 12 g of O 2. a. Which reactant is the limiting reagent? b. Which is the excess reactant? c. How many grams of Al 2 O 3 are formed? Step 1 Step 2 Step 3 Step 4 Step 5 Step 6 Step 7 Step 8

Your Turn: : Practice Problem 1: Skill 8: Limiting Reactant Step 1 Step 2 Problem: Take the reaction: Hg(s) + S(s) Hg. S(s). In an experiment, 200 g of mercury is allowed to react with 1200 g of S. a. Which reactant is the limiting reagent? b. Which is the excess reactant? c. How many grams of Hg. S are formed? Step 3 Step 4 Step 5 Step 6 Step 7 Step 8

Your Turn: : Practice Problem 1: Skill 8: Limiting Reactant Step 1 Step 2 Step 3 Step 4 When lead (II) nitrate reacts with sodium iodide, sodium nitrate and lead (II) iodide are formed as per the following equation: Pb(NO 3)2 (aq) + Na. I (aq) Pb. I 2 (s) + Na. NO 3 (aq) If you start with 25. 0 grams of lead (II) nitrate and 15. 0 grams of sodium iodide, identify the limiting and excess reagents and predict how many grams of sodium nitrate can be formed. Step 5 Step 6 Step 7 Step 8

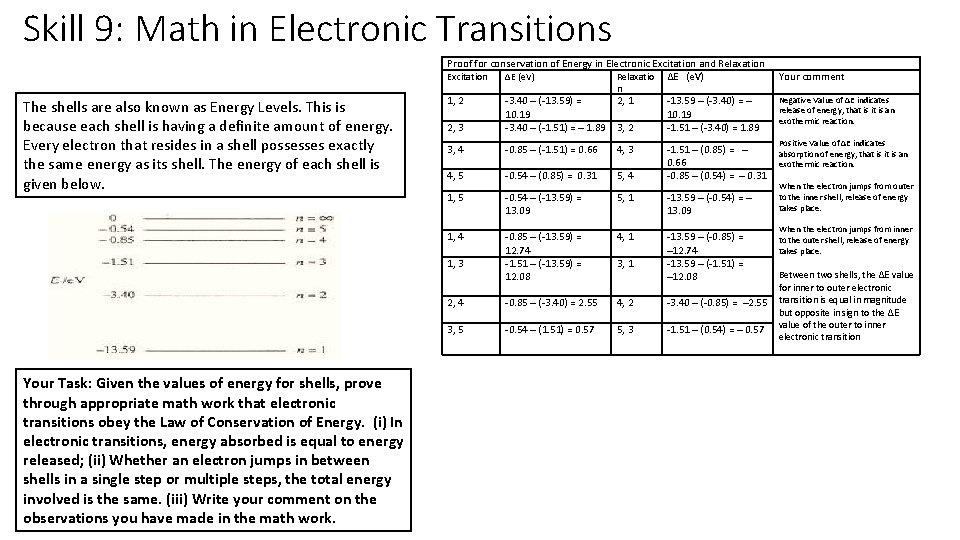
Skill 9: Math in Electronic Transitions Proof for conservation of Energy in Electronic Excitation and Relaxation Excitation ΔE (e. V) Relaxatio ΔE (e. V) The shells are also known as Energy Levels. This is because each shell is having a definite amount of energy. Every electron that resides in a shell possesses exactly the same energy as its shell. The energy of each shell is given below. 1, 2 2, 3 3, 4 4, 5 1, 4 1, 3 2, 4 3, 5 Your Task: Given the values of energy for shells, prove through appropriate math work that electronic transitions obey the Law of Conservation of Energy. (i) In electronic transitions, energy absorbed is equal to energy released; (ii) Whether an electron jumps in between shells in a single step or multiple steps, the total energy involved is the same. (iii) Write your comment on the observations you have made in the math work. -3. 40 ─ (-13. 59) = 10. 19 -3. 40 ─ (-1. 51) = ─ 1. 89 n 2, 1 3, 2 -13. 59 ─ (-3. 40) = ─ 10. 19 -1. 51 ─ (-3. 40) = 1. 89 -0. 85 ─ (-1. 51) = 0. 66 -0. 54 ─ (0. 85) = 0. 31 4, 3 -0. 54 ─ (-13. 59) = 13. 09 -0. 85 ─ (-13. 59) = 12. 74 -1. 51 ─ (-13. 59) = 12. 08 -0. 85 ─ (-3. 40) = 2. 55 -0. 54 ─ (1. 51) = 0. 57 5, 1 -13. 59 ─ (-0. 54) = ─ 13. 09 4, 1 -13. 59 ─ (-0. 85) = ─12. 74 -13. 59 ─ (-1. 51) = ─12. 08 5, 4 3, 1 -1. 51 ─ (0. 85) = ─ 0. 66 -0. 85 ─ (0. 54) = ─ 0. 31 4, 2 -3. 40 ─ (-0. 85) = ─2. 55 5, 3 -1. 51 ─ (0. 54) = ─ 0. 57 Your comment Negative Value of ΔE indicates release of energy, that is it is an exothermic reaction. Positive Value of ΔE indicates absorption of energy, that is it is an exothermic reaction. When the electron jumps from outer to the inner shell, release of energy takes place. When the electron jumps from inner to the outer shell, release of energy takes place. Between two shells, the ΔE value for inner to outer electronic transition is equal in magnitude but opposite in sign to the ΔE value of the outer to inner electronic transition

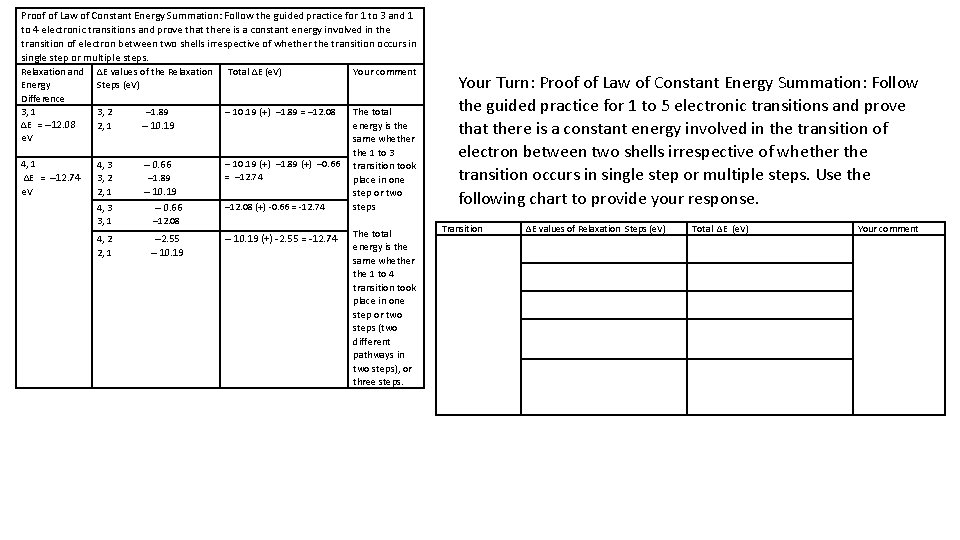
Proof of Law of Constant Energy Summation: Follow the guided practice for 1 to 3 and 1 to 4 electronic transitions and prove that there is a constant energy involved in the transition of electron between two shells irrespective of whether the transition occurs in single step or multiple steps. Relaxation and ΔE values of the Relaxation Total ΔE (e. V) Your comment Energy Steps (e. V) Difference 3, 1 3, 2 ─1. 89 ─ 10. 19 (+) ─1. 89 = ─12. 08 The total ΔE = ─12. 08 2, 1 ─ 10. 19 energy is the e. V same whether the 1 to 3 4, 1 ─ 10. 19 (+) ─1. 89 (+) ─0. 66 transition took 4, 3 ─ 0. 66 = ─12. 74 ΔE = ─12. 74 3, 2 ─1. 89 place in one e. V 2, 1 ─ 10. 19 step or two ─12. 08 (+) -0. 66 = -12. 74 steps 4, 3 ─ 0. 66 3, 1 ─12. 08 4, 2 ─2. 55 ─ 10. 19 (+) -2. 55 = -12. 74 The total energy is the 2, 1 ─ 10. 19 same whether the 1 to 4 transition took place in one step or two steps (two different pathways in two steps), or three steps. Your Turn: Proof of Law of Constant Energy Summation: Follow the guided practice for 1 to 5 electronic transitions and prove that there is a constant energy involved in the transition of electron between two shells irrespective of whether the transition occurs in single step or multiple steps. Use the following chart to provide your response. Transition ΔE values of Relaxation Steps (e. V) Total ΔE (e. V) Your comment

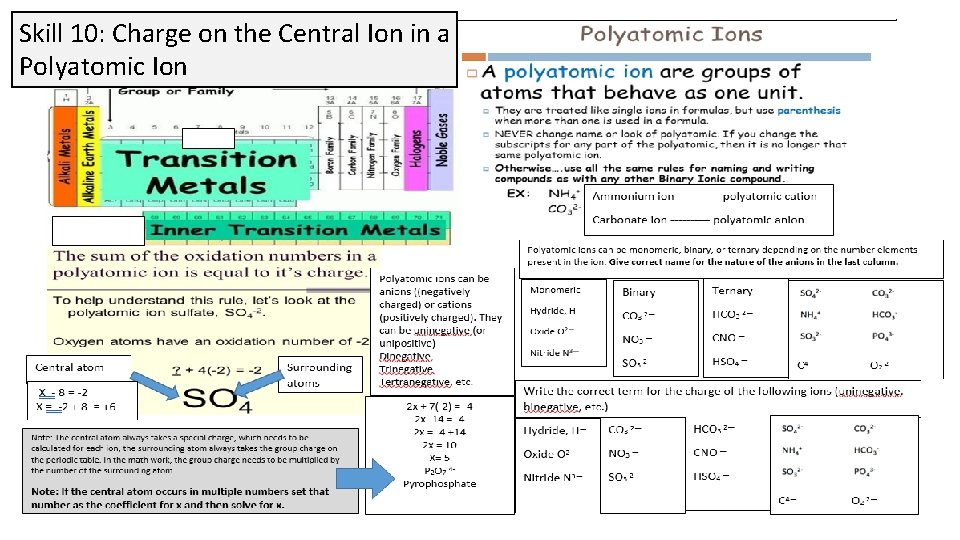
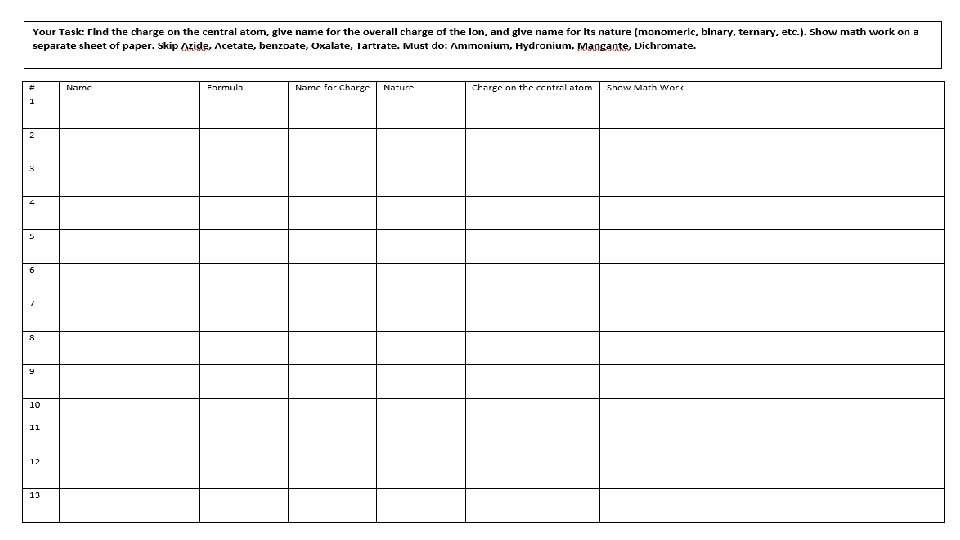
Skill 10: Charge on the Central Ion in a Polyatomic Ion
