MATH OF CHEMISTRY A compound is a substance
MATH OF CHEMISTRY

A compound is a substance composed of two or more different elements that are chemically combined in a fixed proportion. A chemical compound can be broken down by chemical means. Compounds are electrically neutral. J Deutsch 2003 2

A chemical formula is both qualitative and quantitative. It tells which elements are in the compound with symbols and how many of each with subscripts. The formula for sulfuric acid is H 2 SO 4 Element Number of atoms H 2 S 1 O 4 The subscript 1 is never written in a formula J Deutsch 2003 3
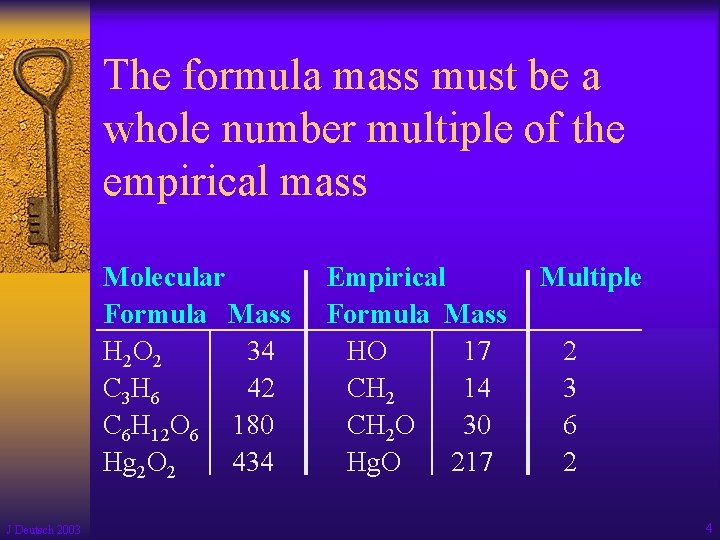
The formula mass must be a whole number multiple of the empirical mass Molecular Formula Mass H 2 O 2 34 C 3 H 6 42 C 6 H 12 O 6 180 Hg 2 O 2 434 J Deutsch 2003 Empirical Formula Mass HO 17 CH 2 14 CH 2 O 30 Hg. O 217 Multiple 2 3 6 2 4

The formula mass of a substance is the sum of the atomic masses of its atoms. The molar mass (gram formula mass) of a substance equals one mole of that substance. (3. 3 e) Formula mass of H 2 SO 4 H 2 x 1 = 2 S 1 x 32 = 32 O 4 x 16 = 64 98 amu J Deutsch 2003 5

The formula mass represents the mass of one molecule of a substance while the gram formula mass represents the 23 mass of a mole (6. 02 x 10 molecules) of that substance. ¨ The calculation formula mass and gram formula mass (GFM) are the same, the difference is in the units. If they tell you to use the mass – Formula Mass of H 2 SO 4 = 98 amu – Gram Formula Mass of H 2 SO 4 = 98 g J Deutsch 2003 rounded to the nearest tenth then use tenths for each mass as well as your final answer. 6

In all chemical reactions there is a conservation of mass, energy, and charge. • Matter cannot be created nor destroyed, only changed from one form to another • Energy cannot be created nor destroyed, only changed from one form to another J Deutsch 2003 7

A balanced chemical equation represents conservation of atoms. • A mole of molecules is made up of 6. 02 x 1023 molecules • A chemical equation is balanced to ensure the conservation of matter/energy, and charge. • The total mass before the reaction must equal the total mass after the reaction has taken place. J Deutsch 2003 8

Chemical equations must be balanced so that mass can be conserved. Word equation: hydrogen + oxygen Chemical equation: 2 H 2 + O 2 J Deutsch 2003 water 2 H 2 O 9

Regents Question: 08/02 #9 If an equation is balanced properly, both sides of the equation must have the same number of (1) atoms (2) coefficients 2 H 2 + O 2 2 H 2 O (3) molecules (4) moles of molecules J Deutsch 2003 10

How many on the left? How many on the right? What do I do? ¨ Balance the reaction Fe + O 2 Fe 2 O 3 – There are 2 oxygen atoms on the left and 3 on the right. To get them equal I need to multiply the left by 3 and the right by 2. These multiples are called coefficients and are placed in front of the formula. The yield sign and the plus separate the formulas. ¨ Fe + 3 O 2 2 Fe 2 O 3 – Now the number of iron atoms has to be balanced. There is one iron on the left and four on the right. Use a coefficient of 4 in front of the Fe on the left. ¨ 4 Fe + 3 O 2 2 Fe 2 O 3 – Now it is correctly balanced J Deutsch 2003 11
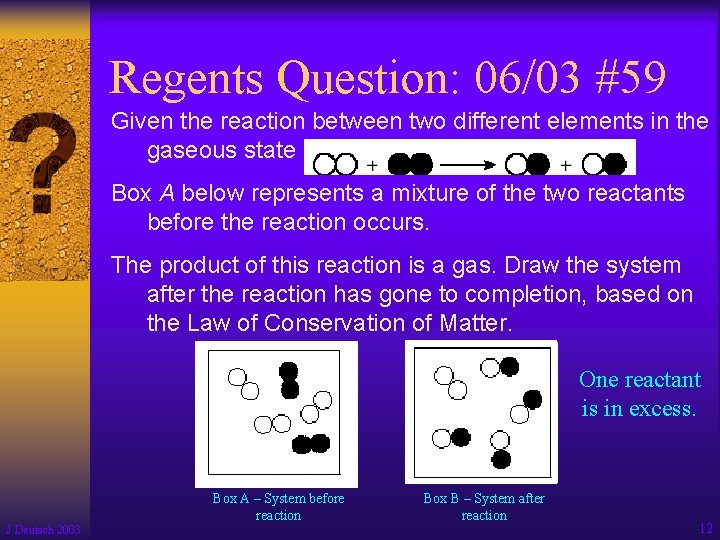
Regents Question: 06/03 #59 Given the reaction between two different elements in the gaseous state Box A below represents a mixture of the two reactants before the reaction occurs. The product of this reaction is a gas. Draw the system after the reaction has gone to completion, based on the Law of Conservation of Matter. One reactant is in excess. J Deutsch 2003 Box A – System before reaction Box B – System after reaction 12

Use the coefficients to predict the amount of reactant consumed or product formed (Mole-Mole problems) ¨ The ratio of the coefficients is a ratio of the moles taking part in a reaction. ¨ Reactants (on the left of the arrow) are consumed ¨ Products (on the right of the arrow) are formed ¨ Given the number of moles of any substance in a reaction, you can use the coefficients to find the number of moles of any other substance. J Deutsch 2003 13
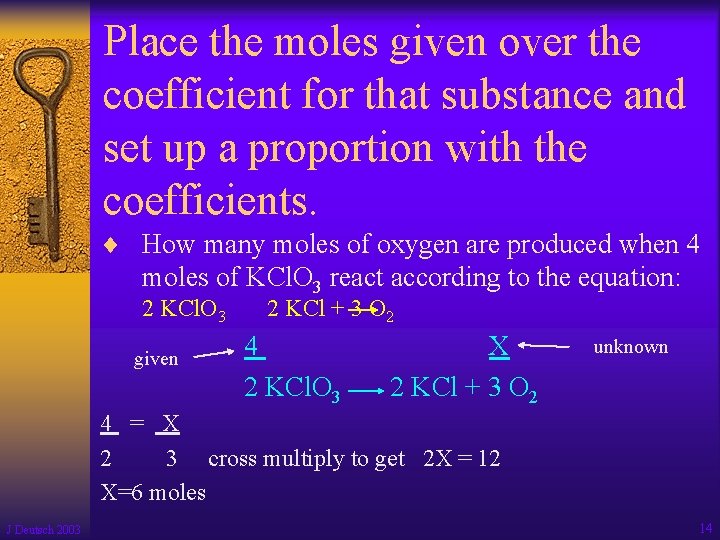
Place the moles given over the coefficient for that substance and set up a proportion with the coefficients. ¨ How many moles of oxygen are produced when 4 moles of KCl. O 3 react according to the equation: 2 KCl. O 3 given 2 KCl + 3 O 2 4 2 KCl. O 3 X 2 KCl + 3 O 2 unknown 4 = X 2 3 cross multiply to get 2 X = 12 X=6 moles J Deutsch 2003 14

Regents Question: 01/03 #42 Given the equation: 2 C 2 H 2(g) + 5 O 2(g) 4 CO 2(g) + 2 H 2 O(g) How many moles of oxygen are required to react completely with 1. 0 mole of C 2 H 2? (1) 2. 5 (2) 2. 0 (3) 5. 0 (4) 10 J Deutsch 2003 15

Regents Question: 06/02 #37 Given the reaction: 6 CO 2 +6 H 2 O C 6 H 12 O 6 + 6 O 2 what is the total number of moles of water needed to make 2. 5 moles of C 6 H 12 O 6 ? (1) 2. 5 (2) 6. 0 (3) 12 (4) 15 J Deutsch 2003 16

Regents Question: 06/03 #20 Given the reaction: Pb. Cl 2 (aq) + Na 2 Cr. O 4 (aq) Pb. Cr. O 4 (s) + 2 Na. Cl(aq) What is the total number of moles of Na. Cl formed when 2 moles of Na 2 Cr. O 4 react completely? (1) 1 mole (2) 2 moles (3) 3 moles (4) 4 moles J Deutsch 2003 17

Regents Question: 06/02 #34 A compound has a gram formula mass of 56 grams per mole. What is the molecular formula for this compound? (1) CH 2 (2) C 2 H 4 (3) C 3 H 6 (4) C 4 H 8 J Deutsch 2003 18

Regents Question: 06/02 #41 The gram formula mass of NH 4 Cl is (1) 22. 4 g/mole (2) 28. 0 g/mole N 1 x 14. 0 = (3) 53. 5 g/mole H 4 x 1. 0 = (4) 95. 5 g/mole Cl 1 x 35. 5 = _______ g/mole J Deutsch 2003 19

Grams/GFM = Moles To convert grams into moles, divide the grams by the gram formula mass To convert moles into grams, multiply by the moles by the gram formula mass The unit for gram formula mass is grams/mole ¨ How many moles in 49 grams of H 2 SO 4? 49 g = X moles 98 g/mol X= 0. 50 moles ¨ How many grams are contained in 2. 00 moles of H 2 SO 4? X = 2. 00 moles 98 g/mol J Deutsch 2003 X = 196 grams 20
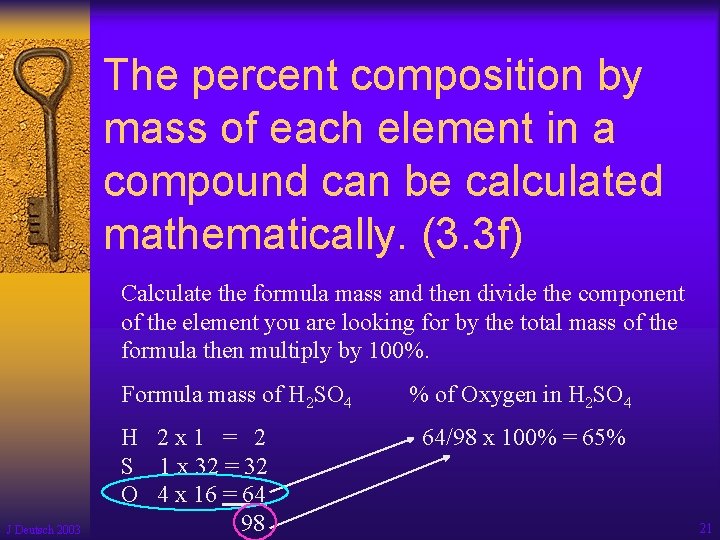
The percent composition by mass of each element in a compound can be calculated mathematically. (3. 3 f) Calculate the formula mass and then divide the component of the element you are looking for by the total mass of the formula then multiply by 100%. Formula mass of H 2 SO 4 J Deutsch 2003 H 2 x 1 = 2 S 1 x 32 = 32 O 4 x 16 = 64 98 % of Oxygen in H 2 SO 4 64/98 x 100% = 65% 21

Regents Question: 01/03 #8 What is the percent by mass of oxygen in H 2 SO 4? [ formula mass = 98] (1) 16% (2) 33% 64/98 x 100 (3) 65% (4) 98% J Deutsch 2003 22

Regents Question: 06/02 #7 The percent by mass of hydrogen in NH 3 is equal to (1) 17 x 100 1 (2) 17 x 100 3 (3) 1 x 100 17 (4) 3 x 100 17 J Deutsch 2003 23

Regents Question: 06/03 #10 The percent by mass of calcium in the compound calcium sulfate (Ca. SO 4 ) is approximately (1) 15% (2) 29% (3) 34% (4) 47% J Deutsch 2003 24

A hydrate is a compound which has water trapped in its crystal structure. We can determine the percentage of water in a hydrate. ¨ Cu. SO 4 5 H 2 O – for every copper(II) sulfate there are 5 trapped water molecules ¨ Find the formula mass including the water – – – J Deutsch 2003 Cu S O H O 1 x 1 x 4 x 10 x 5 x 64 = 64 32 = 32 16 = 64 1 = 10 16 = 80 250 Water contributes 90 to the 250 90/250 x 100% = 36% 25

Regents Question: 06/02 #36 What is the total number of oxygen atoms in the formula Mg. SO 4 • 7 H 2 O? [The • represents seven units of H 2 O attached to one unit of Mg. SO 4] (1) 11 (2) 7 (3) 5 (4) 4 J Deutsch 2003 26
- Slides: 26