Math in Science Accuracy Precision Scientific Notation Math




- Slides: 13

Math in Science Accuracy & Precision | Scientific Notation

Math in Science Learning Objectives • Describe basic fundamentals of math associated with science • Practice math skills utilized in science • Reference mathematical properties and definitions used in science

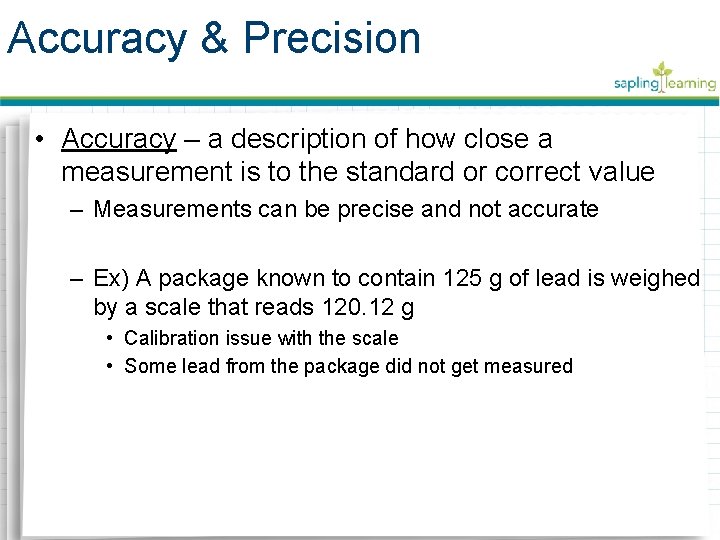
Accuracy & Precision • Precision – a description of the level of exactness of a measurement – Related to the tool used to make the measurement • Specialized instruments have high levels of precision – Micrometer – a device that clamps down on an object to determine its thickness with extreme precision – Caliper – a device used to precisely measure internal or external lengths

Accuracy & Precision • Accuracy – a description of how close a measurement is to the standard or correct value – Measurements can be precise and not accurate – Ex) A package known to contain 125 g of lead is weighed by a scale that reads 120. 12 g • Calibration issue with the scale • Some lead from the package did not get measured

Accuracy & Precision • All measurements have some level of uncertainty – Limitations of instrument – Experimenter using the instrument • Human error is a common source of uncertainty – Limit human error in experiments

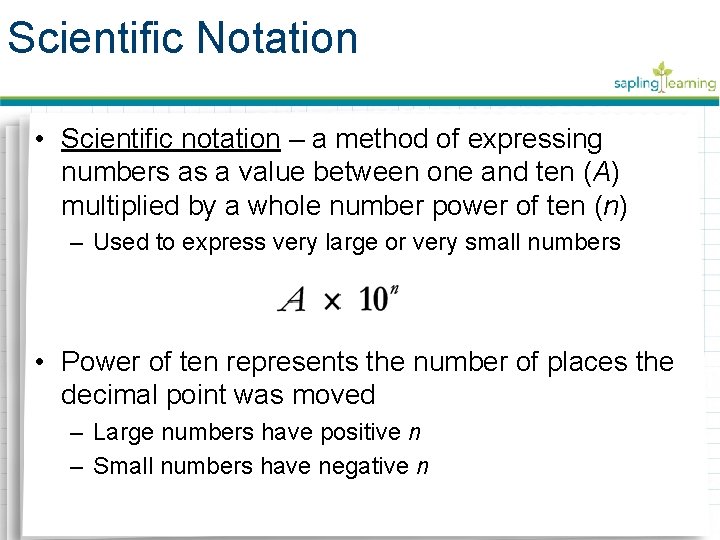
Scientific Notation • Scientific notation – a method of expressing numbers as a value between one and ten (A) multiplied by a whole number power of ten (n) – Used to express very large or very small numbers • Power of ten represents the number of places the decimal point was moved – Large numbers have positive n – Small numbers have negative n

Scientific Notation • Converting a large number into scientific notation Step 1 Count the number of place values from the ones position to the digit preceding the highest or leftmost digit. point the andhighest the following knownby to abe Step 2 Write place digits value that digit, are followed decimalaccurate. timesthe tennumeric raised tovalue, the number place values from Step 3 After write a of multiplication symbol Step 1.

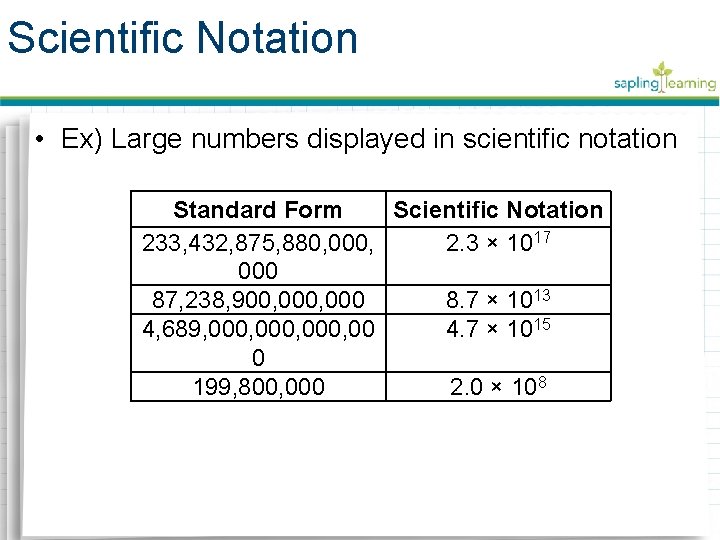
Scientific Notation • Ex) Large numbers displayed in scientific notation Standard Form Scientific Notation 233, 432, 875, 880, 000, 2. 3 × 1017 000 87, 238, 900, 000 8. 7 × 1013 4, 689, 000, 00 4. 7 × 1015 0 199, 800, 000 2. 0 × 108

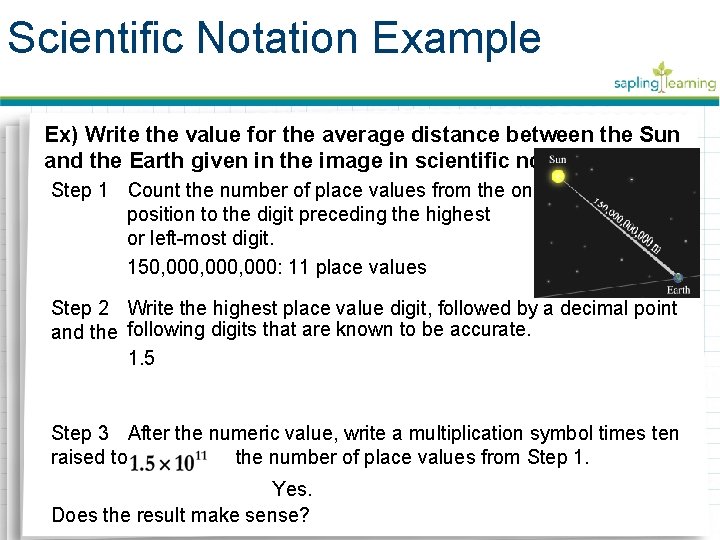
Scientific Notation Example Ex) Write the value for the average distance between the Sun and the Earth given in the image in scientific notation. Step 1 Count the number of place values from the ones position to the digit preceding the highest or left-most digit. 150, 000, 000: 11 place values Step 2 Write the highest place value digit, followed by a decimal point and the following digits that are known to be accurate. 1. 5 Step 3 After the numeric value, write a multiplication symbol times ten raised to the number of place values from Step 1. Yes. Does the result make sense?

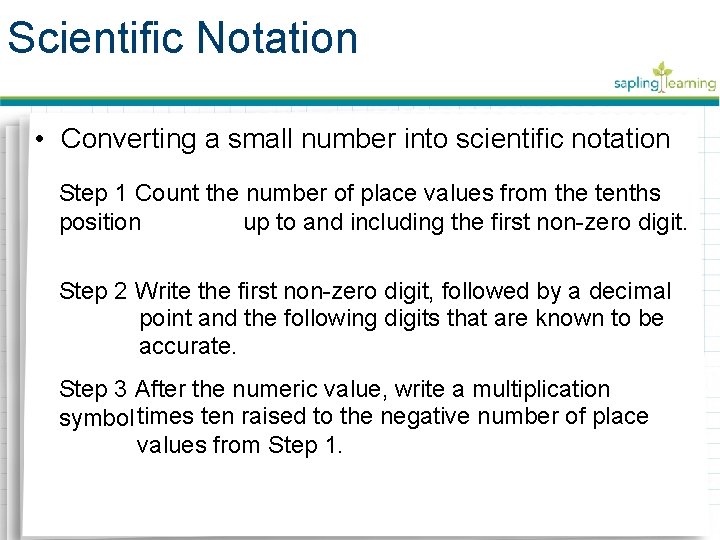
Scientific Notation • Converting a small number into scientific notation Step 1 Count the number of place values from the tenths position up to and including the first non-zero digit. Step 2 Write the first non-zero digit, followed by a decimal point and the following digits that are known to be accurate. Step 3 After the numeric value, write a multiplication symbol times ten raised to the negative number of place values from Step 1.

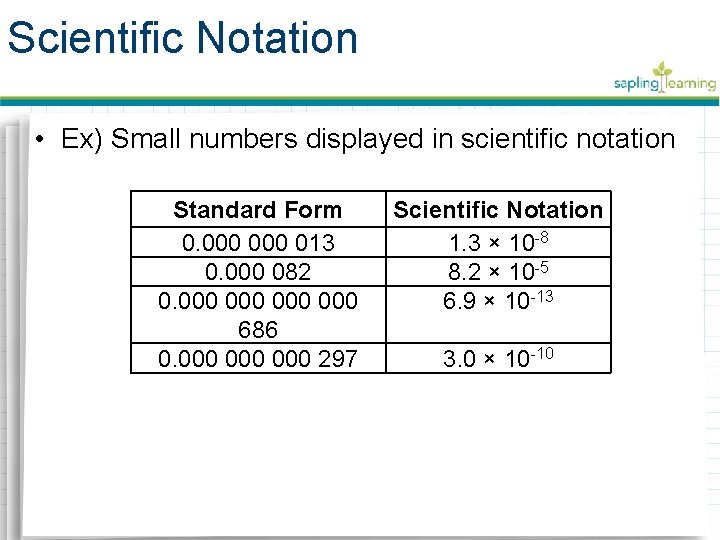
Scientific Notation • Ex) Small numbers displayed in scientific notation Standard Form 0. 000 013 0. 000 082 0. 000 000 686 0. 000 000 297 Scientific Notation 1. 3 × 10 -8 8. 2 × 10 -5 6. 9 × 10 -13 3. 0 × 10 -10

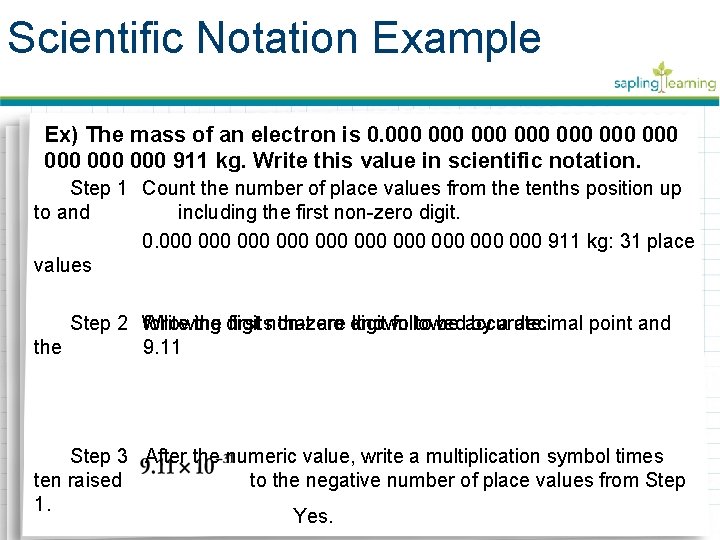
Scientific Notation Example Ex) The mass of an electron is 0. 000 000 000 911 kg. Write this value in scientific notation. Step 1 Count the number of place values from the tenths position up to and including the first non-zero digit. 0. 000 000 000 911 kg: 31 place values Step 2 Write the digits first non-zero followed by a decimal point and following that are digit known to be accurate. the 9. 11 Step 3 After the numeric value, write a multiplication symbol times ten raised to the negative number of place values from Step 1. Yes.

Math in Science Learning Objectives • Describe basic fundamentals of math associated with science • Practice math skills utilized in science • Reference mathematical properties and definitions used in science