Materials used to preserve pulp vitality Calcium hydroxide























- Slides: 25

Materials used to preserve pulp vitality

Calcium hydroxide The characteristics of calcium hydroxide come from its dissociation into calcium and hydroxyl ions. The action of these ions on tissues and bacteria explains the biological and antimicrobial properties of this substance.

it is possible to state that: 1. Dentin is considered the best pulpal protective, and calcium hydroxide has proved, through numerous studies, its capability of inducing the formation of a mineralized bridge over pulpal tissue. 2. It is necessary, whenever possible, to provide time for calcium hydroxide paste to manifest its potential of action on the microorganisms present in endodontic infections. The maintenance of a high concentration of hydroxyl ions can change bacteria enzymatic activity and promote its inactivation.

3. The site of action of hydroxyl ions of calcium hydroxide includes the enzymes in the cytoplasmic membrane. This medication has a large scope of action, and therefore is effective on a wide range of microorganisms, regardless their metabolic capability. In microbial world, cytoplasmic membranes are similar, irrespective from microorganisms morphological, tinctorial and respiratory characteristics, which means that this medication has a similar effect on aerobic, anaerobic, Grampositive and Gram-negative bacteria.

4. Calcium hydroxide as temporary dressing used between appointments promotes better results on the periapical healing process than the treatment in one appointment. Calcium hydroxide encourages the deposition of a hard tissue bridge that usually protects the dental pulp. The ability to stimulate mineralization associated to the antimicrobial effectiveness confers on it the current success as an endodontic medication.

calcium hydroxide is an excellent therapeutic option when the clinical situation requires the use of pulp capping agent and intracanal medication. Two effects of this medication need to be considered, biological and antimicrobial effects. Thus, others factors can alter this effects, like the influence of vehicles in this properties and the time of action for to express microbial control. Other substances with objectives similar to calcium hydroxide, like Mineral Trioxide Agregate and Portland cement, have also been studied

In the dental pulp, calcium hydroxide has been used as a pulp capping agent because of its ability to stimulate mineralization; as intracanal dressing it has excellent antimicrobial action, that favor to eliminate microorganisms after cleaning and shaping, to neutralize the remaining toxins, besides maintaining the temporary sealing.

The chemical dynamics of calcium hydroxide as demonstrated by ionic dissociation, characterizes its properties. The activation of tissue enzymes such as alkaline phosphatase shows mineralizing effects and inhibiting effect on bacterial enzymes, which leads to its antimicrobial property, illustrating the biological qualities of hydroxyl and calcium ions on both tissue and microorganisms

BIOLOGICAL EFFECT Calcium hydroxide is a strong base obtained through calcination (heating) of calcium carbonate until its transformation into calcium oxide. Calcium hydroxide is obtained through the hydration of calcium oxide and the chemical reaction between calcium hydroxide and carbon dioxide forms calcium carbonate. It is a white powder with a high p. H (12. 6) and is slightly soluble in water (solubility of 1. 2 g/L, at a temperature of 25 o. C)36.

The properties of calcium hydroxide come from its dissociation into calcium and hydroxyl ions and the action of these ions on tissues and bacteria explains biological and antimicrobial properties of this substance. Changes in the biological properties can also be understood through the chemical reactions, since calcium hydroxide, in the presence of carbon dioxide, becomes calcium carbonate (weak acid oxide) and this product does not have calcium hydroxide's biological properties such as the mineralizing capability.

MICROBIAL EFFECT The mechanism of action of calcium hydroxide on microorganisms can be explained by the influence of p. H on growth, metabolism and bacterial cell division. the hydroxyl ions from calcium hydroxide develop their mechanism of action in the cytoplasmic membrane, because enzymatic sites are located in the cytoplasmic membrane

This membrane is responsible for essential functions such as metabolism, cellular division and growth and it takes part in the final stages of cellular wall formation, biosynthesis of lipids, transport of electrons and oxidative phosphorylation. Extracellular enzymes act on nutrients, carbohydrates, proteins, and lipids that, through hydrolysis, favor digestion. Intracellular enzymes located in the cell favor respiratory activity of the cellular wall structure. The p. H gradient of the cytoplasmic membrane is altered by the high concentration of hydroxyl ions of calcium hydroxide acting on the proteins of the membrane (proteic denaturation).

The effect of the high p. H of calcium hydroxide alters the integrity of the cytoplasmic membrane by means of chemical injury to organic components and transport of nutrients, or by means of the destruction of phospholipids or unsaturated fatty acids of the cytoplasmic membrane, observed in the peroxidation process, which is a saponification reaction

Using calcium hydroxide as a pulpcapping materials Vital pulp capping is the dressing of an exposed pulp with the aim of maintaining pulp vitality. Throughout the life of a tooth, vital pulp tissue contributes to the production of secondary dentin, peritubular dentin (sclerosis) and reparative dentin in response to biologic and pathologic stimuli.

The pulp tissue , with its circulation extending into the tubular dentin , keeps the dentin moist, which in turn ensures that the dentin maintains its resilience and toughness. These characteristics ensure that the teeth can successfully resist the forces of mastication.

Direct pulp capping should be used only on a vital pulp that has been accidentally injured and shows no other symptoms. Direct pulp capping should not be performed on a pulp that has been exposed as a result of penetrating caries. A successful pulp cap has a vital pulp and a dentin bridge within 75 to 90 days.

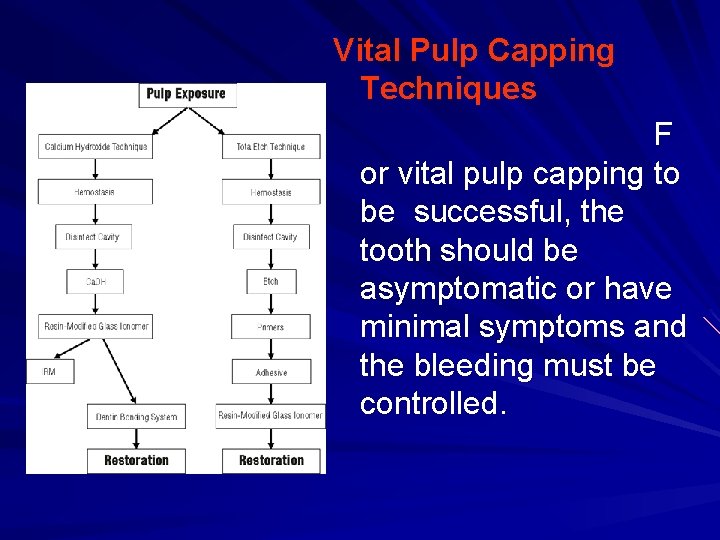
Vital Pulp Capping Techniques F or vital pulp capping to be successful, the tooth should be asymptomatic or have minimal symptoms and the bleeding must be controlled.

This control may be achieved by washing the area with sterile saline and drying it with either paper points or cotton pellets, by using cotton pellets soaked with hydrogen peroxide or 5. 25% sodium hypochlorite, or, if necessary, by using a hemostatic agent such as Hemodent (Premier Dental Products, Norristown, Pa. ). If bleeding fails to stop after two or three attempts, then endodontic therapy should be considered. Several studies have indicated that the size of the perforation is less important than obtaining hemostasis.

Following hemostasis, a disinfectant (e. g. , Cavity Cleanser, Ultradent Products Inc. , ) should be placed on the cavity floor. The area is then air dried, and calcium hydroxide in a formula such as Dycal (Dentsply Canada Ltd. , ), Life (Kerr Manufacturing, ) or Ultradent Calcium Hydroxide (Ultradent Products Inc. , ) is placed directly in contact with pulp tissue. This step is very important, for the better the contact of the calcium hydroxide dressing with the pulpal wound, the better the healing.

The calcium hydroxide should then be covered with a resin-modified glass ionomer extended onto dentin. Subsequently, a permanent restoration can be placed, with a dentin bonding system used to seal the margins of the restoration. An alternative is to place a zinc oxide-eugenol restoration over the calcium hydroxide cap. Zinc oxide-eugenol provides an excellent seal and, with its anti-microbial properties, makes for a very good temporary restoration. After three months, assuming pulp vitality and no symptoms, the zinc oxide-eugenol can be removed and a more permanent sealed restoration placed.

For the total etch procedure, as with calcium hydroxide, hemostasis must be obtained. The exposure site is then covered with a non-setting calcium hydroxide paste (e. g. , Pulpdent, ) and the cavity preparation completed. Following disinfection of the cavity, the enamel and dentin are etched with 32% phosphoric acid for 15 seconds. The acid and calcium hydroxide are rinsed off and the preparation is lightly dried.

The entire preparation , including enamel, dentin and pulpal tissue , is treated with a dentin bonding system (a fourthgeneration system with a separate primer and adhesive is recommended, as little research has been published to date on the fifth-generation dentin bonding systems). Following placement of several layers of the hydrophilic primer, a thin layer of the adhesive resin is painted onto the enamel, dentin and pulpal tissue and light cured.

A second layer of unfilled resin is applied, and a thin layer of resin-modified glass ionomer is also applied over and around the exposure site to mechanically protect the perforation from intrusion of the restorative material during packing or condensation. These layers are also light cured. The restoration is subsequently completed in conventional fashion.

Conclusion Mechanical exposures are more likely than carious exposures to be successfully capped. If the operator properly selects the case, obtains hemostasis, disinfects the exposure and the cavity preparation, and adequately seals the exposure and the cavity preparation, success can be obtained with either the calcium hydroxide technique or the total etch technique.

Although both techniques can achieve successful vital pulp caps, the calcium hydroxide technique has demonstrated its success over a longer period of time. Which technique offers the better prognosis awaits the results of many more long-term studies.