MATERIALS SCIENCE LESSON 1 CHEM 30 ATTENDANCE INTRO













































- Slides: 60

MATERIALS SCIENCE LESSON 1

CHEM 30 • ATTENDANCE • INTRO TO ME • COURSE OUTLINE/FINAL PROJECT • CLASS WEBSITE • WHO ARE YOU SHEETS • START UNIT 1

WHO AM I?

CHEMISTRY 30 SYLLABUS

• MY EMAIL: APPLETONE@SPSD. SK. CA CLASS WEBSITE: WWW. EASTLYNAPPLETON. COM

COURSE DESCRIPTION A MAJOR FOCUS OF THE COURSE IS THE STUDY OF THE ROLE OF CHEMICAL PROPERTIES AND BONDS IN DETERMINING WHAT MAKES MATERIALS SUITABLE FOR USE IN SPECIFIC APPLICATIONS. STUDENTS WILL ACTIVELY INVESTIGATE THE NATURE OF EQUILIBRIUM IN CHEMICAL REACTIONS. IN ELECTROCHEMISTRY, STUDENTS EXPLORE OXIDATION-REDUCTION REACTIONS AND THE IMPACT OF ELECTROCHEMISTRY ON SOCIETY AND THE ENVIRONMENT. OTHER TOPICS INCLUDE ORGANIC COMPOUNDS AND ACID-BASE CHEMISTRY. STUDENT INQUIRY WILL GUIDE INDEPENDENT INVESTIGATIONS OF CHEMISTRYRELATED PHENOMENA.

PREREQUISITE • PHYSICAL SCIENCE 20 OR CHEMISTRY 20

OUTCOMES • STUDENT DIRECTED STUDY: • MATERIALS SCIENCE • CHEMICAL EQUILIBRIA • ELECTROCHEMISTRY

ASSESSMENT • ASSIGNMENTS/HOMEWORK:

• QUIZZES:

• EXAMS: • MISSED EXAMS • REWRITES • MIDTERM/FINAL

• LABS • HOW TO PROPERLY REFERENCE A LAB FOR MATERIALS AND PROCEDURE: • APPLETON, E. (2010). CHEM 30 MATERIALS SCIENCE. LAB ONE: IDENTIFYING SOLIDS (PP. 9 -11). SASKATOON, SK: NUTANA COLLEGIATE.

• OVERALL: • I WANT YOU TO BE SUCCESSFUL IN THIS CLASS. I BELIEVE IN SECOND CHANCES AND OFFER MANY. IF THERE IS SOMETHING I CAN DO TO HELP YOU TO SUCCEED PLEASE LET ME KNOW.

BEHAVIOUR EXPECTATIONS • NO EATING OR DRINKING DURING LAB PERIODS. • STUDENTS ARE EXPECTED TO ATTEND ALL CLASSES. IF A STUDENT MISSES CLASS, THEY MUST LET THE SCHOOL KNOW THE REASON (CALL 683 -7580, EMAIL NCIATTENDANCE@GMAIL. COM OR TEXT 260 -2118). IF A STUDENT MISSES CLASS HE/SHE WILL HAVE TO MAKE UP THE MISSED WORK. THERE WILL BE A HOMEWORK BINDER IN CLASS WHERE STUDENTS WILL BE ABLE TO FIND EXTRA COPIES OF MISSED HANDOUTS, HOWEVER, IT WILL BE THE STUDENT’S RESPONSIBILITY TO COPY MISSED NOTES. IF YOU HAVE ACCESS TO A COMPUTER, YOU MAY EMAIL ME TO GET MISSED WORK. MY EMAIL ADDRESS IS APPLETONE@SPSD. SK. CA.

• ALL STUDENTS MUST BE ON TIME FOR CLASS. THERE WILL BE QUIZZES AT THE BEGINNING OF MANY OF OUR CLASSES WHICH YOU WILL MISS IF YOU ARE LATE AND THE DOOR IS CLOSED, PLEASE KNOCK ON THE DOOR AND WAIT OUTSIDE UNTIL SOMEONE COMES TO LET YOU IN. IF THE DOOR IS OPEN, PLEASE JOIN THE CLASS AS QUIETLY AS POSSIBLE. • TALKING WILL BE ALLOWED DURING MOST WORK TIME BUT IT MUST NOT DISTURB OTHER STUDENTS. YOU MAY LISTEN TO YOUR IPOD/MP 3/WALKMAN WHILE WORKING ON ASSIGNMENTS; HOWEVER IPODS ETC. SHOULD NEVER BE USED WHILE I AM SPEAKING OR DURING QUIZZES/EXAMS.

• ASSIGNMENTS WILL BE DUE ON THE DAY OF THE UNIT EXAM (WHICH WILL USUALLY BE NEGOTIATED AS A CLASS). IF YOU FEEL THAT YOU WILL BE UNABLE TO FINISH AN ASSIGNMENT BY ITS DUE DATE, PLEASE CONTACT ME AHEAD OF TIME AND, IN MOST CASES, AN EXTENSION WILL BE GRANTED. • STUDENTS SHOULD NOT LEAVE THE CLASS WITHOUT TALKING TO ME FIRST. I NEED TO KNOW WHERE YOU ARE.

• EXTRA HELP: • MORNINGS AT 9: 00 AM • TUTORIAL AT LUNCH • TWO HELPFUL PLACES TO WATCH HELPFUL VIDEOS ARE KHAN ACADEMY AND CRASH COURSE (MAYBE WRITE THIS DOWN)

BIBLIOGRAPHY:

EVALUATIVE ASSESSMENT PIECES

RESEARCH PROJECT

• AUDIO CONSENT FORM- 16, 17, 18

CLASS WEBSITE • HTTP: //WWW. EASTLYNAPPLETON. COM/

• WHO ARE YOU?

MATERIALS SCIENCE: BONDING MS 1: Lesson 1 Outcome: Examine the role of valence electrons in the formation of chemical bonds Indicators: Trace the historical development of the model of the atom. Examine how evidence and experimentation inform the development and refinement of theories in chemistry Explain the relationship between the position of an element on the periodic table and its number of valence electrons with reference to the octet rule. Explain the formation of ions and predict their charge in group 1 and 2 elements and non‐metals, based on an understanding of valence electrons and the octet rule. Draw Lewis structures (electron dot structures) for group 1 and 2 elements and non‐metals, based on an understanding of valence electrons.

WHAT IS CHEMISTRY?

CHEMISTRY INTRO • WHAT IS CHEMISTRY? • CHEMISTRY IS THE STUDY OF THE COMPOSITION AND PROPERTIES OF MATTER. • MATTER HAS TWO GENERAL PROPERTIES; IT OCCUPIES SPACE AND HAS MASS.

WHY STUDY CHEMISTRY? • CHEMISTRY CAN HELP TO EXPLAIN HOW THINGS WORK. • CHEMISTRY MAY HELP YOU IN YOUR FUTURE CAREER. EVEN IF YOU ARE NOT PLANNING ON BEING A CHEMIST, MANY JOBS REQUIRE A BASIC UNDERSTANDING OF CHEMISTRY. • CHEMISTRY CAN HELP YOU TO BE AN INFORMED CITIZEN. KNOWLEDGE OF CHEMISTRY CAN HELP YOU TO EVALUATE DATA, ARRIVE AT AN INFORMED OPINION AND TAKE APPROPRIATE ACTION.

TOWER CHALLENGE ASSIGNMENT

THE SCIENTIFIC METHOD: • SCIENTIFIC METHOD IS THE PROCESS THAT SCIENTISTS FOLLOW IN ORDER TO PERFORM RESEARCH TO INVESTIGATE THE WORLD AROUND THEM. • THE SCIENTIFIC METHOD: • GATHER INFORMATION THROUGH _________ • DEFINE THE _________ • CREATE A ________ • _________ AN EXPERIMENT TO TEST THE HYPOTHESIS • ________ AND OBSERVE THE EXPERIMENT • _________ DATA FROM EXPERIMENT • MODIFY OF CREATE NEW _____________ • ANALYZE, DESIGN, HYPOTHESIS, OBSERVATION, PERFORM, QUESTION

THE SCIENTIFIC METHOD: • SCIENTIFIC METHOD IS THE PROCESS THAT SCIENTISTS FOLLOW IN ORDER TO PERFORM RESEARCH TO INVESTIGATE THE WORLD AROUND THEM. • THE SCIENTIFIC METHOD: • GATHER INFORMATION THROUGH OBSERVATION • DEFINE THE QUESTION • CREATE A HYPOTHESIS • DESIGN AN EXPERIMENT TO TEST THE HYPOTHESIS • PERFORM AND OBSERVE THE EXPERIMENT • ANALYZE DATA FROM EXPERIMENT • MODIFY OF CREATE NEW HYPOTHESIS Q 7 of the Tower Challenge: How was the process you went through with this task similar to the classic “scientific method”?

MODEL OF THE ATOM • HTTP: //THEHISTORYOFTHEATOM. WEEBLY. COM/


• WHY DO WE STUDY THE HISTORY OF THE ATOM?

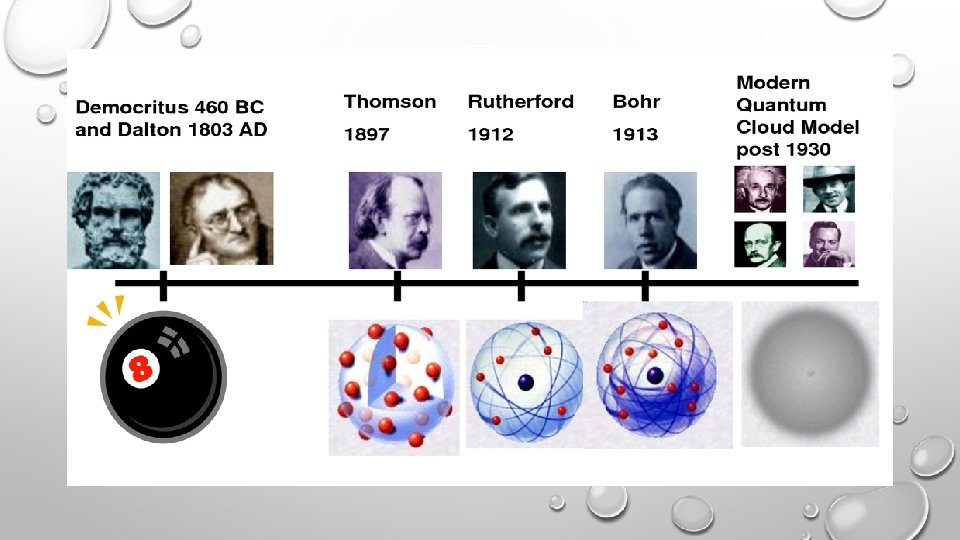
DEMOCRITUS • LIVED FROM: 460 -370 BC PUT FORWARD ATOMIC MODEL IN: 442 BC DESCRIPTION OF HIS MODEL: • DEMOCRITUS’S MODEL STATED THAT MATTER CONSISTS OF INVISIBLE PARTICLES CALLED ATOMS AND A VOID (EMPTY SPACE). • HE STATED THAT ATOMS ARE INDESTRUCTIBLE AND UNCHANGEABLE. • ALSO THAT THEY ARE HOMOGENOUS, MEANING THEY HAVE NO INTERNAL STRUCTURE. • HIS ATOMIC MODEL WAS SOLID, AND STATED ALL ATOMS DIFFER IN SIZE, SHAPE, MASS, POSITION AND ARRANGEMENT, WITH A VOID EXISTS BETWEEN THEM.

DEMOCRITUS • DEMOCRITUS KNEW THAT IF YOU TOOK A STONE AND CUT IT IN HALF, EACH HALF HAD THE SAME PROPERTIES AS THE ORIGINAL STONE. HE REASONED THAT IF YOU CONTINUED TO CUT THE STONE INTO SMALLER AND SMALLER PIECES, AT SOME POINT YOU WOULD REACH A PIECE SO TINY THAT IT COULD NO LONGER BE DIVIDED. • ARISTOTLE AND PLATO, TWO OF THE BEST-KNOWN PHILOSOPHERS OF ANCIENT GREECE, REJECTED THEORIES OF DEMOCRITUS. ARISTOTLE ACCEPTED THEORY OF EMPEDOCLES (EMPEDOCLES ARGUED THAT ALL MATTER WAS COMPOSED OF FOUR ELEMENTS: FIRE, AIR, WATER, AND EARTH. THE RATIO OF THESE FOUR ELEMENTS AFFECTED THE PROPERTIES OF THE MATTER). ARISTOTLE ADDED HIS OWN (INCORRECT) IDEA THAT THE FOUR CORE ELEMENTS COULD BE TRANSFORMED INTO ONE ANOTHER. • BECAUSE OF ARISTOTLE'S GREAT INFLUENCE, DEMOCRITUS' THEORY WOULD HAVE TO WAIT ALMOST 2, 000 YEARS BEFORE BEING REDISCOVERED.

JOHN DALTON • LIVED FROM: 1766 -1844 PUT FORWARD ATOMIC MODEL IN: 1803 NICKNAME FOR HIS MODEL: BILLIARD BALL MODEL DESCRIPTION OF HIS MODEL: JOHN DALTON WAS AN ENGLISH CHEMIST. HIS IDEAS FROM "THE ATOMIC THEORY OF MATTER. "

JOHN DALTON • HERE ARE HIS IDEAS: • MATTER CONSISTS OF INDIVISIBLE ATOMS. • ALL OF THE ATOMS OF A GIVEN CHEMICAL ELEMENT ARE IDENTICAL IN MASS AND IN ALL OTHER PROPERTIES. • DIFFERENT CHEMICAL ELEMENTS HAVE DIFFERENT KINDS OF ATOMS; IN PARTICULAR, THEIR ATOMS HAVE DIFFERENT MASSES. • ATOMS ARE INDESTRUCTIBLE AND RETAIN THEIR IDENTITIES IN CHEMICAL REACTIONS. • A COMPOUND FORMS FROM ITS ELEMENTS THROUGH THE COMBINATION OF ATOMS OF UNLIKE ELEMENTS IN SMALL WHOLE NUMBER RATIOS, SUCH AS 1 TO 1, 2 TO 2, 2 TO 3, AND SO ON (RATIO OF WATER IS 2 HYDROGEN ATOMS TO 1 OXYGEN ATOM).

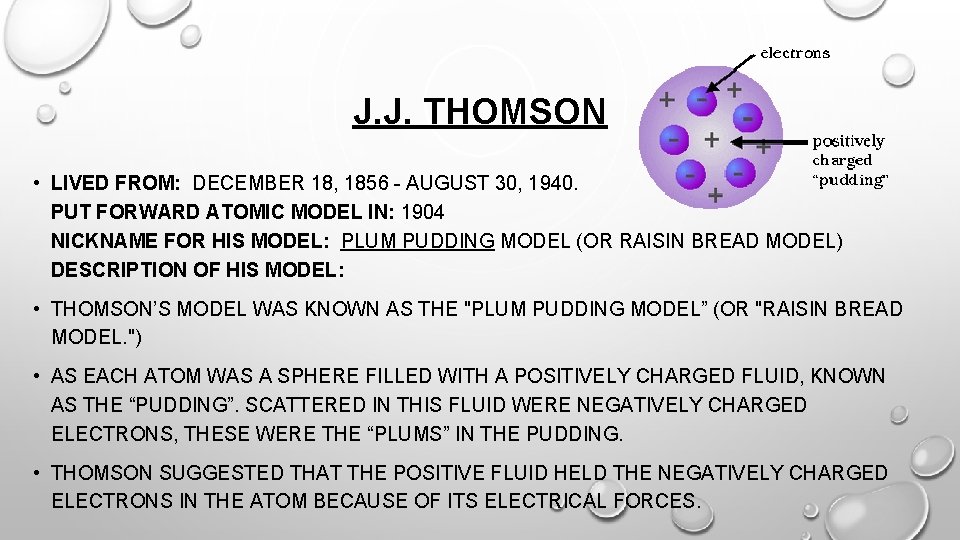
J. J. THOMSON • LIVED FROM: DECEMBER 18, 1856 - AUGUST 30, 1940. PUT FORWARD ATOMIC MODEL IN: 1904 NICKNAME FOR HIS MODEL: PLUM PUDDING MODEL (OR RAISIN BREAD MODEL) DESCRIPTION OF HIS MODEL: • THOMSON’S MODEL WAS KNOWN AS THE "PLUM PUDDING MODEL” (OR "RAISIN BREAD MODEL. ") • AS EACH ATOM WAS A SPHERE FILLED WITH A POSITIVELY CHARGED FLUID, KNOWN AS THE “PUDDING”. SCATTERED IN THIS FLUID WERE NEGATIVELY CHARGED ELECTRONS, THESE WERE THE “PLUMS” IN THE PUDDING. • THOMSON SUGGESTED THAT THE POSITIVE FLUID HELD THE NEGATIVELY CHARGED ELECTRONS IN THE ATOM BECAUSE OF ITS ELECTRICAL FORCES.

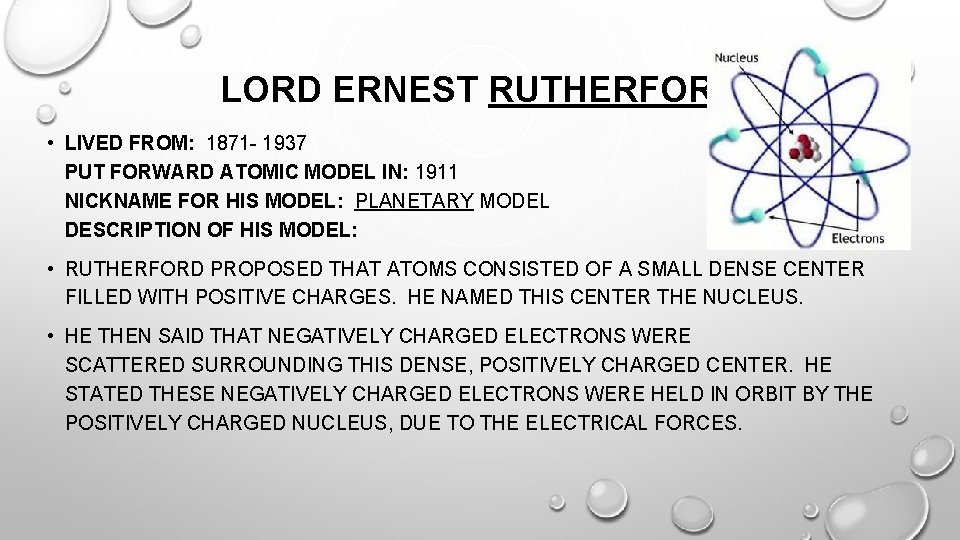
LORD ERNEST RUTHERFORD • LIVED FROM: 1871 - 1937 PUT FORWARD ATOMIC MODEL IN: 1911 NICKNAME FOR HIS MODEL: PLANETARY MODEL DESCRIPTION OF HIS MODEL: • RUTHERFORD PROPOSED THAT ATOMS CONSISTED OF A SMALL DENSE CENTER FILLED WITH POSITIVE CHARGES. HE NAMED THIS CENTER THE NUCLEUS. • HE THEN SAID THAT NEGATIVELY CHARGED ELECTRONS WERE SCATTERED SURROUNDING THIS DENSE, POSITIVELY CHARGED CENTER. HE STATED THESE NEGATIVELY CHARGED ELECTRONS WERE HELD IN ORBIT BY THE POSITIVELY CHARGED NUCLEUS, DUE TO THE ELECTRICAL FORCES.

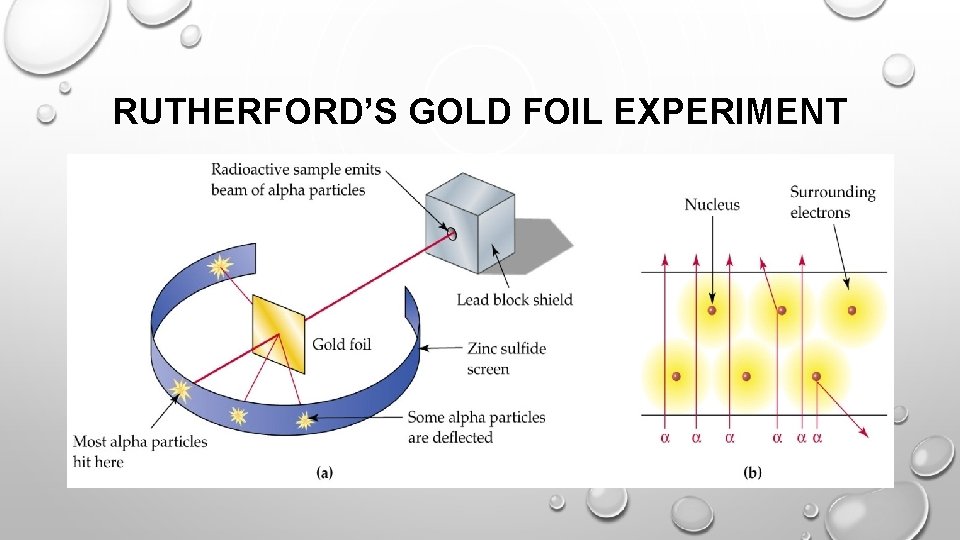
RUTHERFORD • RUTHERFORD PERFORMED A SERIES OF EXPERIMENTS WITH RADIOACTIVE ALPHA PARTICLES. RUTHERFORD FIRED TINY ALPHA PARTICLES AT SOLID OBJECTS SUCH AS GOLD FOIL. HE FOUND THAT WHILE MOST OF THE ALPHA PARTICLES PASSED RIGHT THROUGH THE GOLD FOIL, A SMALL NUMBER OF ALPHA PARTICLES PASSED THROUGH AT AN ANGLE (AS IF THEY HAD BUMPED UP AGAINST SOMETHING) AND SOME BOUNCED STRAIGHT BACK LIKE A TENNIS BALL HITTING A WALL. RUTHERFORD'S EXPERIMENTS SUGGESTED THAT GOLD FOIL, AND MATTER IN GENERAL, HAD HOLES IN IT! THESE HOLES ALLOWED MOST OF THE ALPHA PARTICLES TO PASS DIRECTLY THROUGH, WHILE A SMALL NUMBER RICOCHETED OFF OR BOUNCED STRAIGHT BACK BECAUSE THEY HIT A SOLID OBJECT.

RUTHERFORD’S GOLD FOIL EXPERIMENT

MAX PLANCK • 1900 • PLANCK'S WORK IN THERMODYNAMICS LED TO THE FORMULATIONS OF HIS QUANTUM THEORY. TO EXPLAIN THE COLORS OF HOT GLOWING MATTER, HE PROPOSED THAT ENERGY IS RADIATED IN VERY MINUTE AND DISCRETE QUANTIZED AMOUNTS OR PACKETS, RATHER THAN IN A CONTINUOUS UNBROKEN WAVE. PLANCK CALLED THE PACKETS OF ENERGY QUANTA.

ALBERT EINSTEIN • 1905 • ALBERT EINSTEIN USED PLANCK'S QUANTUM THEORY TO DESCRIBE THE PARTICLE PROPERTIES OF LIGHT. EINSTEIN DEMONSTRATED THAT ELECTROMAGNETIC RADIATION, INCLUDING LIGHT, HAS THE CHARACTERISTICS OF BOTH A WAVE AND, CONSISTENT WITH PLANCK'S THEORY, A PARTICLE. THESE PARTICLES WERE LATER CALLED PHOTONS. • WITH EINSTEIN’S CALCULATIONS, ONE COULD DETERMINE THE SIZE OF THESE INVISIBLE ATOMS AND MOLECULES.

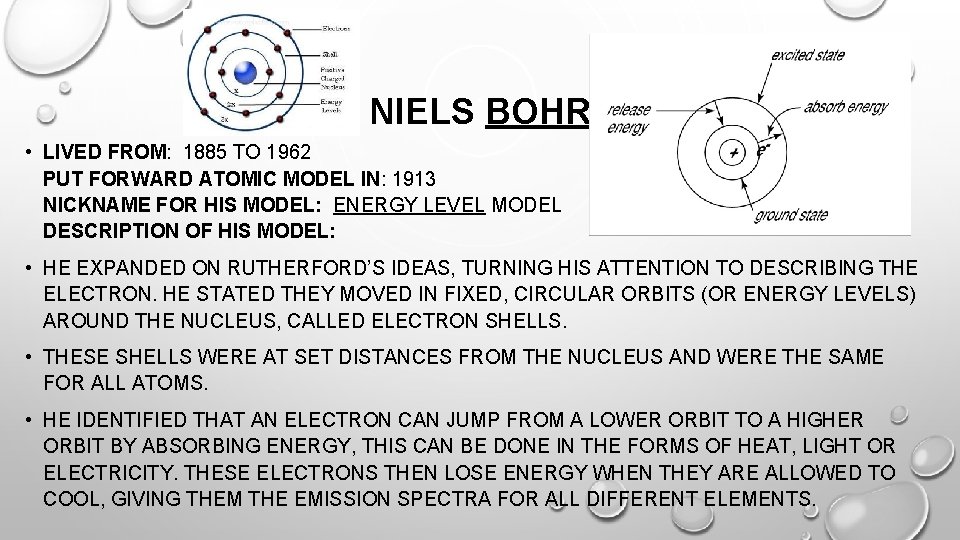
NIELS BOHR • LIVED FROM: 1885 TO 1962 PUT FORWARD ATOMIC MODEL IN: 1913 NICKNAME FOR HIS MODEL: ENERGY LEVEL MODEL DESCRIPTION OF HIS MODEL: • HE EXPANDED ON RUTHERFORD’S IDEAS, TURNING HIS ATTENTION TO DESCRIBING THE ELECTRON. HE STATED THEY MOVED IN FIXED, CIRCULAR ORBITS (OR ENERGY LEVELS) AROUND THE NUCLEUS, CALLED ELECTRON SHELLS. • THESE SHELLS WERE AT SET DISTANCES FROM THE NUCLEUS AND WERE THE SAME FOR ALL ATOMS. • HE IDENTIFIED THAT AN ELECTRON CAN JUMP FROM A LOWER ORBIT TO A HIGHER ORBIT BY ABSORBING ENERGY, THIS CAN BE DONE IN THE FORMS OF HEAT, LIGHT OR ELECTRICITY. THESE ELECTRONS THEN LOSE ENERGY WHEN THEY ARE ALLOWED TO COOL, GIVING THEM THE EMISSION SPECTRA FOR ALL DIFFERENT ELEMENTS.

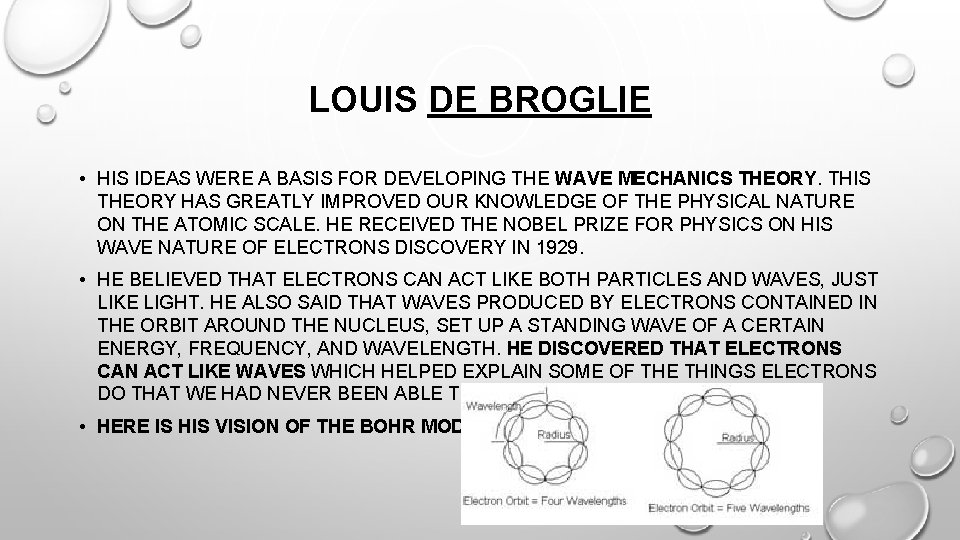
LOUIS DE BROGLIE • HIS IDEAS WERE A BASIS FOR DEVELOPING THE WAVE MECHANICS THEORY. THIS THEORY HAS GREATLY IMPROVED OUR KNOWLEDGE OF THE PHYSICAL NATURE ON THE ATOMIC SCALE. HE RECEIVED THE NOBEL PRIZE FOR PHYSICS ON HIS WAVE NATURE OF ELECTRONS DISCOVERY IN 1929. • HE BELIEVED THAT ELECTRONS CAN ACT LIKE BOTH PARTICLES AND WAVES, JUST LIKE LIGHT. HE ALSO SAID THAT WAVES PRODUCED BY ELECTRONS CONTAINED IN THE ORBIT AROUND THE NUCLEUS, SET UP A STANDING WAVE OF A CERTAIN ENERGY, FREQUENCY, AND WAVELENGTH. HE DISCOVERED THAT ELECTRONS CAN ACT LIKE WAVES WHICH HELPED EXPLAIN SOME OF THE THINGS ELECTRONS DO THAT WE HAD NEVER BEEN ABLE TO EXPLAIN BEFORE. • HERE IS HIS VISION OF THE BOHR MODEL:

• ERWIN SCHRÖDINGER & WERNER HEISENBERG DATE: LATE 1920’S QUICK SUMMARY: • SCHRODINGER & HEISENBERG ESSENTIALLY HAD THE SAME THEORY. • THEY DISCOVERED THAT ELECTRONS DON'T MOVE IN ORBITS (OR IN A SET PATH AT ALL), BUT MOVE IN WAVES, AND THEY HAVE NO EXACT LOCATION. • MATHEMATICAL EQUATIONS COULD DESCRIBE THE LIKELIHOOD OF FINDING AN ELECTRON IN A CERTAIN POSITION. • THIS ATOMIC MODEL IS KNOWN AS THE QUANTUM MECHANICAL MODEL OF THE ATOM. • THE QUANTUM MECHANICAL MODEL PREDICTS THE ODDS OF THE LOCATION OF THE ELECTRON. THIS MODEL CAN BE PORTRAYED AS A NUCLEUS SURROUNDED BY AN ELECTRON CLOUD. • WHERE THE CLOUD IS MOST DENSE, THE PROBABILITY OF FINDING THE ELECTRON IS GREATEST, AND CONVERSELY, THE ELECTRON IS LESS LIKELY TO BE IN A LESS DENSE AREA OF THE CLOUD. THUS, THIS MODEL INTRODUCED THE CONCEPT OF

JAMES CHADWICK • DATE: 1932 QUICK SUMMARY: • JAMES CHADWICK'S ATOMIC THEORY LED TO THE DISCOVERY OF NEUTRONS. • BEFORE HE DISCOVERED THIS, PEOPLE THOUGHT THE ATOM WAS MADE UP OF ONLY PROTONS AND ELECTRONS. THE PROTONS WERE LARGE AND BUNDLED TOGETHER IN THE NUCLEUS, AND THE ELECTRONS WENT AROUND THE NUCLEUS IN A CIRCLE. • THE NEUTRON WAS VERY HARD TO FIND BECAUSE IT DID NOT REPEL THE PROTONS WHEN IT WAS IN THE ATOM. • WITH THE DISCOVERY OF THE NEUTRON, AN ADEQUATE MODEL OF THE ATOM BECAME AVAILABLE TO CHEMISTS.

• SEE ASSIGNMENT Q 1 & 2

ABOUT THE PERIODIC TABLE • WHAT IS THE PERIODIC TABLE? • A WAY TO ORGANIZE ELEMENTS • CONTAINS ALL ELEMENTS EVER DISCOVERED OR CREATED

DEVELOPMENT OF THE PERIODIC TABLE: • THE PERIODIC TABLE WAS DEVELOPED BY DMITRI MENDELEEV (RUSSIAN CHEMIST). HE RECOGNIZED TRENDS IN PROPERTIES OF ELEMENTS WHEN ORGANIZED BY ATOMIC MASS. • HE USED THESE TRENDS TO ORGANIZE THE PERIODIC TABLE. • HE WAS THEN ABLE TO USE THE PERIODIC TABLE TO PREDICT THE PROPERTIES OF ELEMENTS THAT HAD NOT YET BEEN DISCOVERED OR CREATED.

HOW IS THE PERIODIC TABLE ORGANIZED? • THE PERIODIC TABLE IS ORGANIZED BY INCREASING ATOMIC NUMBER. • IT IS ORGANIZED INTO FAMILIES AND PERIODS ARE THE HORIZONTAL ROWS OF THE PERIODIC TABLE, AND FAMILIES (OR GROUPS) ARE THE VERTICAL ROWS OF THE PERIODIC TABLE. MOST ELEMENTS IN A FAMILY HAVE SIMILAR PROPERTIES.

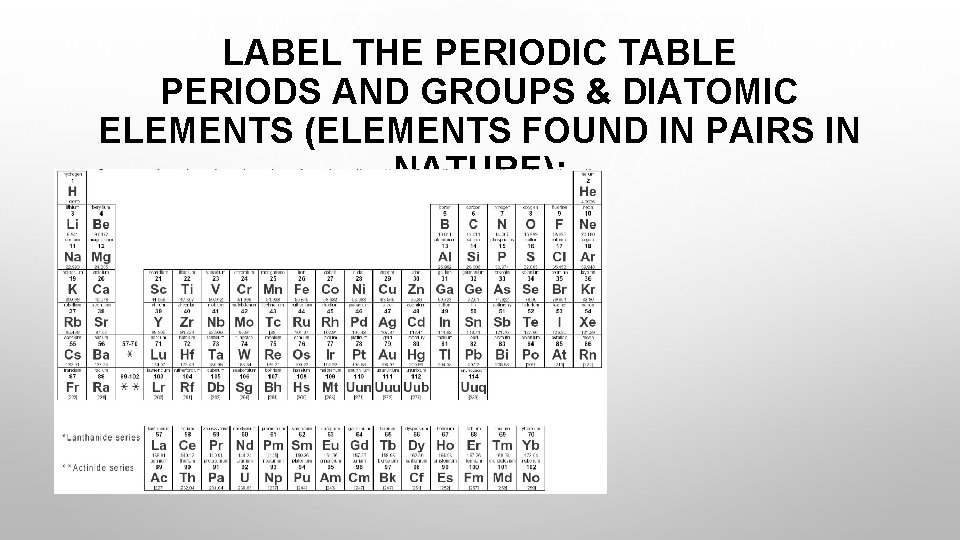
LABEL THE PERIODIC TABLE PERIODS AND GROUPS & DIATOMIC ELEMENTS (ELEMENTS FOUND IN PAIRS IN NATURE):

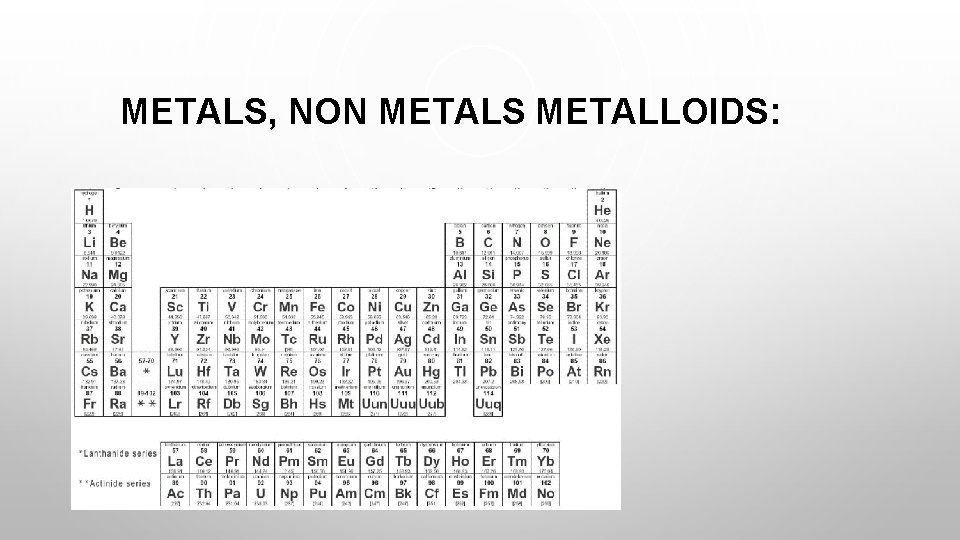
METALS, NON METALS METALLOIDS:

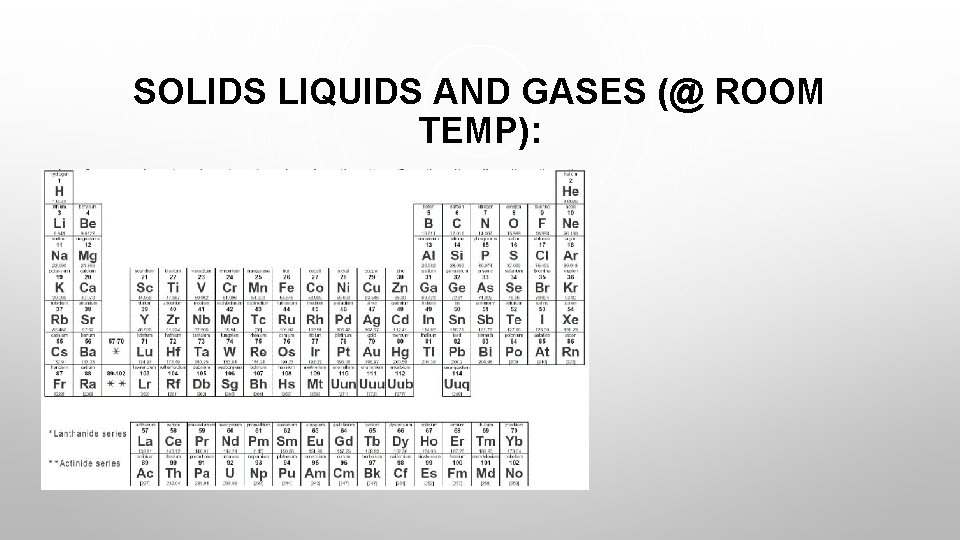
SOLIDS LIQUIDS AND GASES (@ ROOM TEMP):

VALENCE ELECTRONS • WHAT ARE THEY? • VALENCE ELECTRONS ARE THOSE THAT ARE IN THE OUTERMOST ELECTRON SHELL (VALENCE SHELL) OF AN ATOM. THE VALENCE SHELLS ARE THE OUTERMOST S AND P ORBITALS ARE THE HIGHEST ENERGY ORBITALS OF AN ATOM. • VALENCE ELECTRONS DETERMINE HOW AN ELEMENT REACTS WITH OTHER ELEMENTS. • THE FEWER VALENCE ELECTRONS AN ATOM HAS, THE LESS STABLE IT IS AND THE MORE LIKELY IT IS TO REACT. • THE MORE VALENCE ELECTRONS AN ATOM HAS, THE MORE STABLE IT IS AND THE LESS LIKELY IT IS TO REACT. SOMETHING THAT IS UNLIKELY TO REACT IS CALLED INERT.

• OCTET RULE: • THE MOST VALENCE ELECTRONS AN ATOM CAN HAVE IS 8, BECAUSE THAT IS A FULL VALENCE (2 ELECTRONS IN THE S ORBITAL, 6 ELECTRONS IN THE P ORBITAL). ALL ATOMS WOULD LIKE TO HAVE 8 VALENCE ELECTRONS BECAUSE THEN THEY ARE STABLE. THIS IS CALLED THE OCTET RULE (OR THE RULE OF 8). • EXCEPTIONS: • HYDROGEN AND HELIUM CAN ONLY HAVE 2 ELECTRONS IN THEIR VALENCE SHELL (1 S). • TRANSITION METALS (+ A FEW OTHERS) ARE ALSO EXCEPTIONS BECAUSE ORBITALS CAN HYBRIDIZE. WE WILL NOT BE LEARNING ABOUT HYBRIDIZED ORBITALS IN THIS COURSE. • NOTE: 8 VALENCE ELECTRONS AND 0 VALENCE ELECTRONS BOTH MAKE FOR STABLE ATOMS BECAUSE WITH 0 VALENCE ELECTRONS, THEY ESSENTIALLY DO HAVE 8 BECAUSE THE NEXT VALENCE SHELL BELOW THE ONE THAT WAS EMPTIED IS FULL…

• EASY WAY TO DETERMINE NUMBER OF VALENCE ELECTRONS: • GROUP 1 HAS 1 VALENCE ELECTRON, GROUP 2 HAS 2 VALENCE ELECTRONS, GROUPS 3 TO 12 ARE TRANSITION METALS SO WE DON’T NEED TO DETERMINE THE NUMBER OF VALENCE ELECTRONS THEY HAVE, GROUP 13 HAS 3 VALENCE ELECTRONS, GROUP 14 HAS 4 VALENCE ELECTRONS, GROUP 15 HAS ___ ELECTRONS, GROUP 16 HAS ____ ELECTRONS, GROUP 17 HAS _____ ELECTRONS, GROUP 18 HAS ____ ELECTRONS.

• Lewis dot diagrams • Electron-dot formula method or Lewis Formula method is used to represent the number of electrons in the valence shell. • The core is represented by the symbol for the element; valence electrons are represented by dots. • The symbol is assumed to have four sides and the valence electrons are distributed around the sides. • When we distribute valence electrons, we first place one dot on each of the four sides before we locate pairs of electrons on any one side. Usually no more than two electrons can be placed on any one side.

• EXAMPLES: • CALCIUM SELENIUM BROMINE

ASSIGNMENT • COLUMNS 1 AND 4 OF THE VALENCE ELECTRON ASSIGNMENT