Materials Oriented Modelling Catalysis and Interactions in Solid







- Slides: 32

Materials Oriented Modelling - Catalysis and Interactions in Solid and Condensed Phases Stockholm, Sweden on 28 June - 1 July, 2009 1

Background: The tautomerization of cytosine presents unsolved questions This process can only occur with the aid of WATER. Ergo: water can be an important catalyst Materials Oriented Modelling - Catalysis and Interactions in Solid and Condensed Phases Stockholm, Sweden on 28 June - 1 July, 2009 2

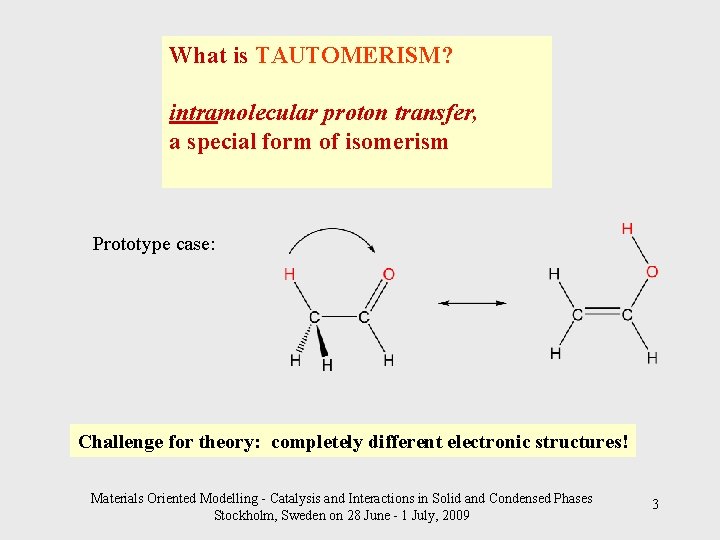
What is TAUTOMERISM? intramolecular proton transfer, a special form of isomerism Prototype case: Challenge for theory: completely different electronic structures! Materials Oriented Modelling - Catalysis and Interactions in Solid and Condensed Phases Stockholm, Sweden on 28 June - 1 July, 2009 3

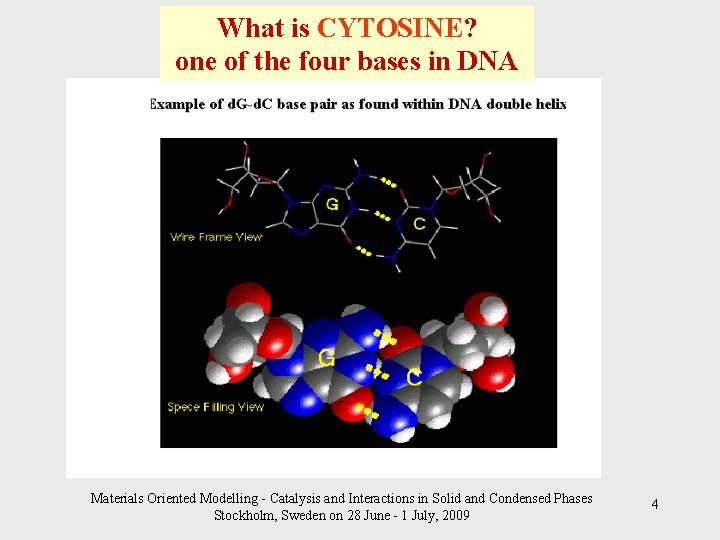
What is CYTOSINE? one of the four bases in DNA Materials Oriented Modelling - Catalysis and Interactions in Solid and Condensed Phases Stockholm, Sweden on 28 June - 1 July, 2009 4

Significance in biological systems: All four nucleotide bases are prototypes of molecules with many possible tautomeric forms If tautomerization occurs, this would totally destroy the hydrogen bond structure of the double helix mutation Materials Oriented Modelling - Catalysis and Interactions in Solid and Condensed Phases Stockholm, Sweden on 28 June - 1 July, 2009 5

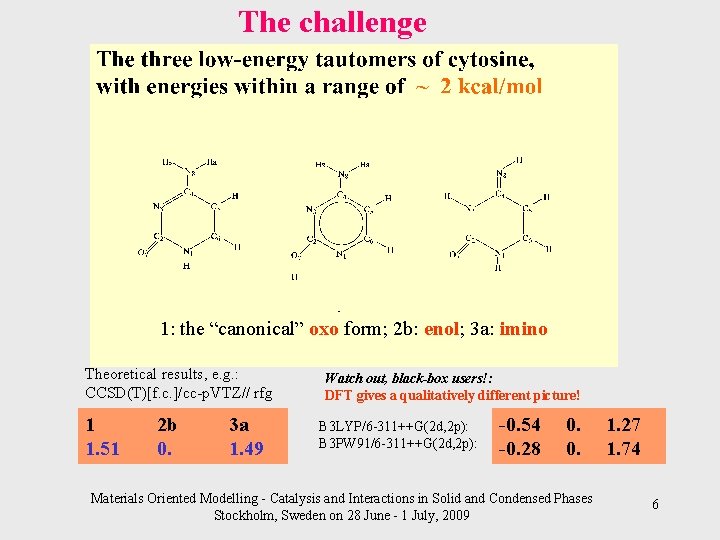
The challenge 1: the “canonical” oxo form; 2 b: enol; 3 a: imino Theoretical results, e. g. : CCSD(T)[f. c. ]/cc-p. VTZ// rfg 1 1. 51 2 b 0. 3 a 1. 49 Watch out, black-box users!: DFT gives a qualitatively different picture! B 3 LYP/6 -311++G(2 d, 2 p): B 3 PW 91/6 -311++G(2 d, 2 p): -0. 54 -0. 28 0. 0. Materials Oriented Modelling - Catalysis and Interactions in Solid and Condensed Phases Stockholm, Sweden on 28 June - 1 July, 2009 1. 27 1. 74 6

Section 1. Test calculations Check the reliability of several methods on small molecules. Test calculations on three systems: Materials Oriented Modelling - Catalysis and Interactions in Solid and Condensed Phases Stockholm, Sweden on 28 June - 1 July, 2009 7

Acetaldehyde Vinylalcohol Materials Oriented Modelling - Catalysis and Interactions in Solid and Condensed Phases Stockholm, Sweden on 28 June - 1 July, 2009 8

Acetaldimine Vinylamine Materials Oriented Modelling - Catalysis and Interactions in Solid and Condensed Phases Stockholm, Sweden on 28 June - 1 July, 2009 9

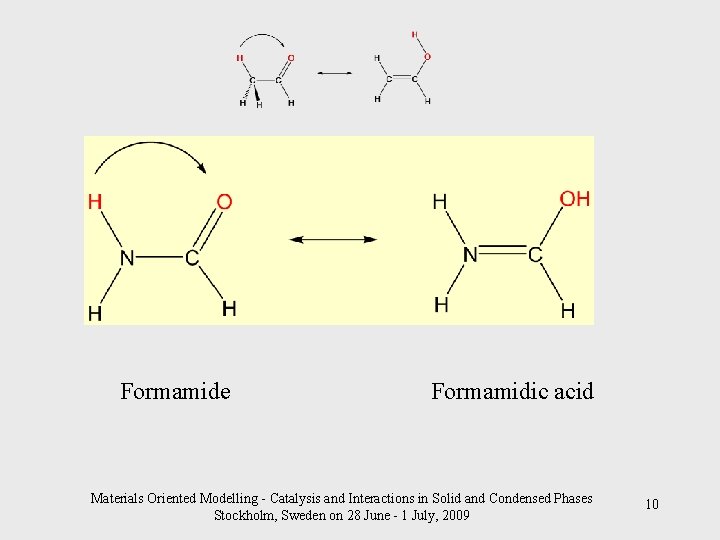
Formamide Formamidic acid Materials Oriented Modelling - Catalysis and Interactions in Solid and Condensed Phases Stockholm, Sweden on 28 June - 1 July, 2009 10

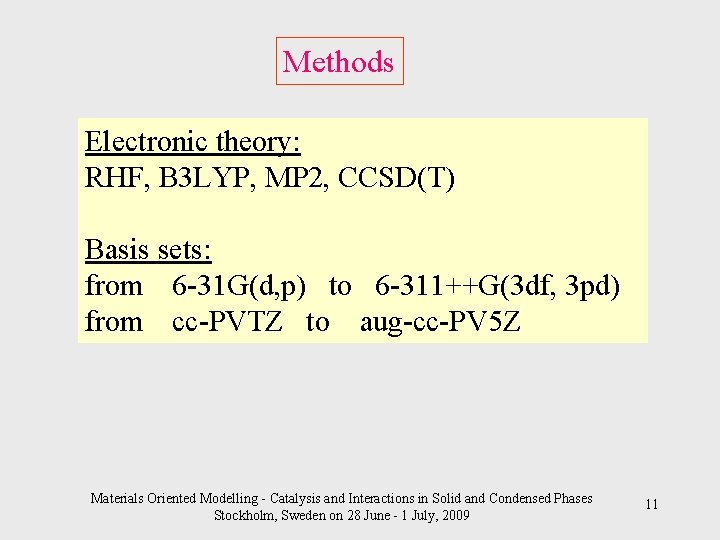
Methods Electronic theory: RHF, B 3 LYP, MP 2, CCSD(T) Basis sets: from 6 -31 G(d, p) to 6 -311++G(3 df, 3 pd) from cc-PVTZ to aug-cc-PV 5 Z Materials Oriented Modelling - Catalysis and Interactions in Solid and Condensed Phases Stockholm, Sweden on 28 June - 1 July, 2009 11

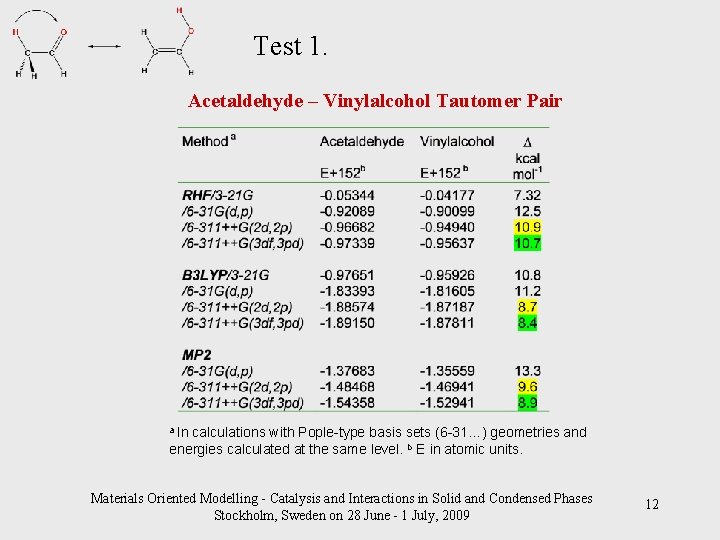
Test 1. Acetaldehyde – Vinylalcohol Tautomer Pair a In calculations with Pople-type basis sets (6 -31…) geometries and energies calculated at the same level. b E in atomic units. Materials Oriented Modelling - Catalysis and Interactions in Solid and Condensed Phases Stockholm, Sweden on 28 June - 1 July, 2009 12

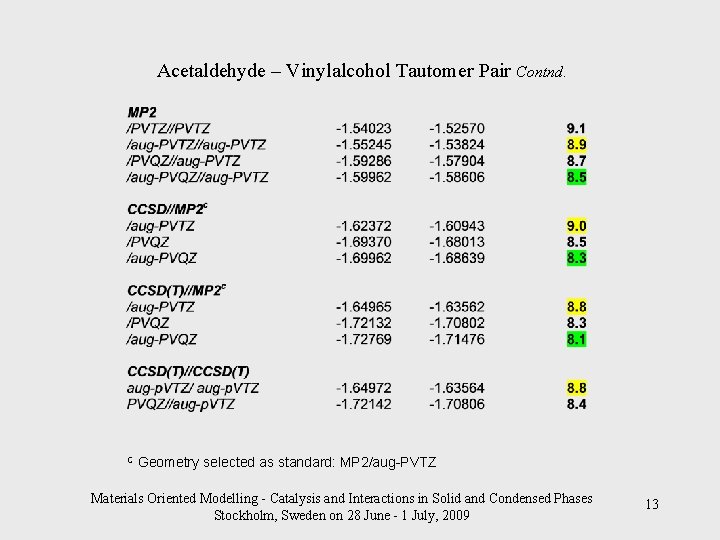
Acetaldehyde – Vinylalcohol Tautomer Pair Contnd. C Geometry selected as standard: MP 2/aug-PVTZ Materials Oriented Modelling - Catalysis and Interactions in Solid and Condensed Phases Stockholm, Sweden on 28 June - 1 July, 2009 13

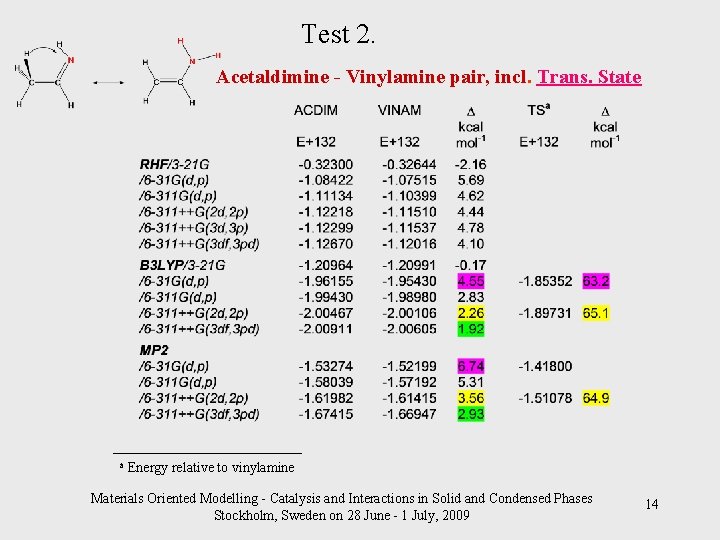
Test 2. Acetaldimine - Vinylamine pair, incl. Trans. State _____________________ a Energy relative to vinylamine Materials Oriented Modelling - Catalysis and Interactions in Solid and Condensed Phases Stockholm, Sweden on 28 June - 1 July, 2009 14

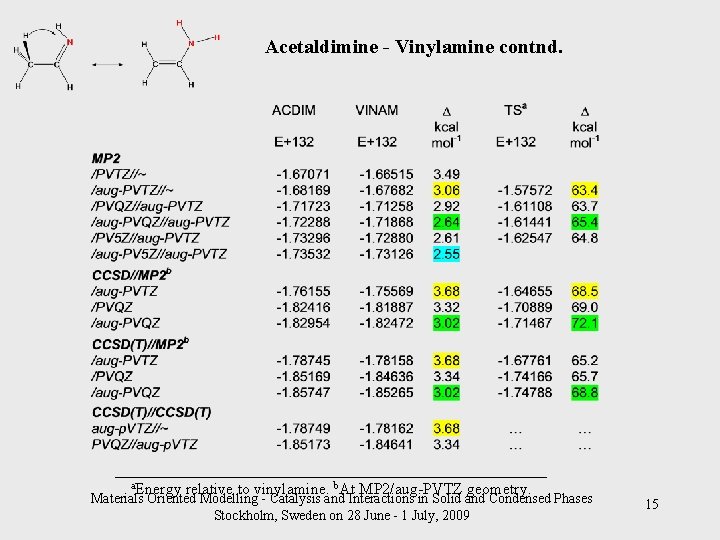
Acetaldimine - Vinylamine contnd. ___________________________ a. Energy relative to vinylamine. b. At MP 2/aug-PVTZ geometry. Materials Oriented Modelling - Catalysis and Interactions in Solid and Condensed Phases Stockholm, Sweden on 28 June - 1 July, 2009 15

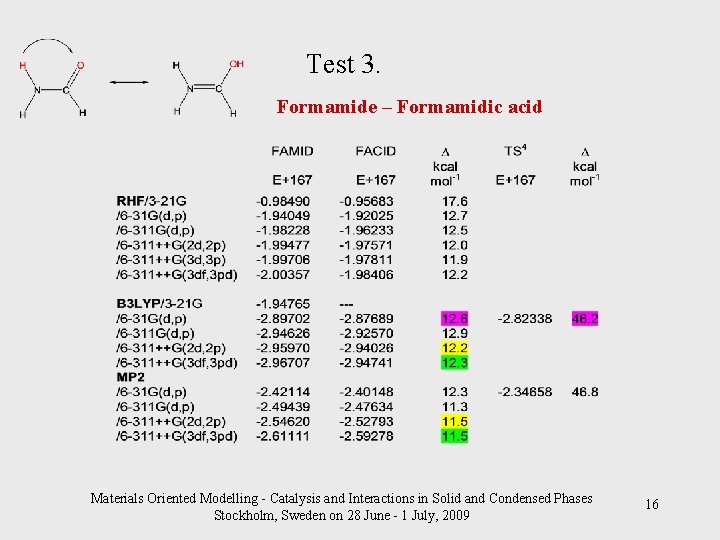
Test 3. Formamide – Formamidic acid Materials Oriented Modelling - Catalysis and Interactions in Solid and Condensed Phases Stockholm, Sweden on 28 June - 1 July, 2009 16

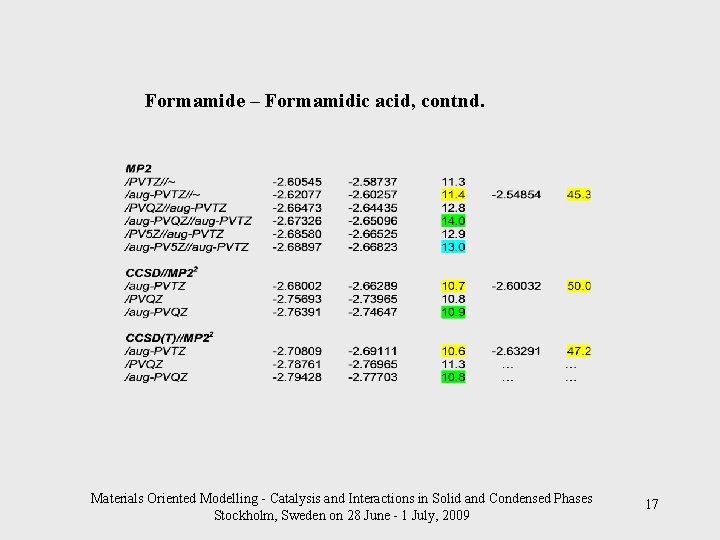
Formamide – Formamidic acid, contnd. Materials Oriented Modelling - Catalysis and Interactions in Solid and Condensed Phases Stockholm, Sweden on 28 June - 1 July, 2009 17

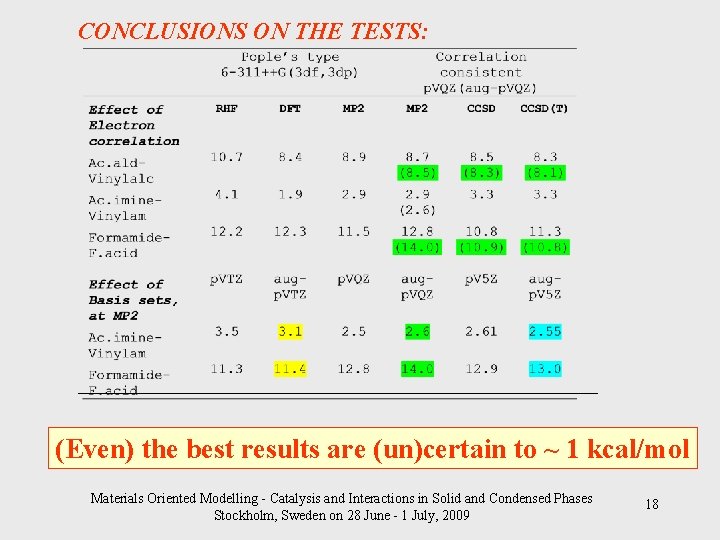
CONCLUSIONS ON THE TESTS: _________________________________ (Even) the best results are (un)certain to ~ 1 kcal/mol Materials Oriented Modelling - Catalysis and Interactions in Solid and Condensed Phases Stockholm, Sweden on 28 June - 1 July, 2009 18

Section 2. The effect of water Model: supermolecule, a water molecule added explicitly Materials Oriented Modelling - Catalysis and Interactions in Solid and Condensed Phases Stockholm, Sweden on 28 June - 1 July, 2009 19

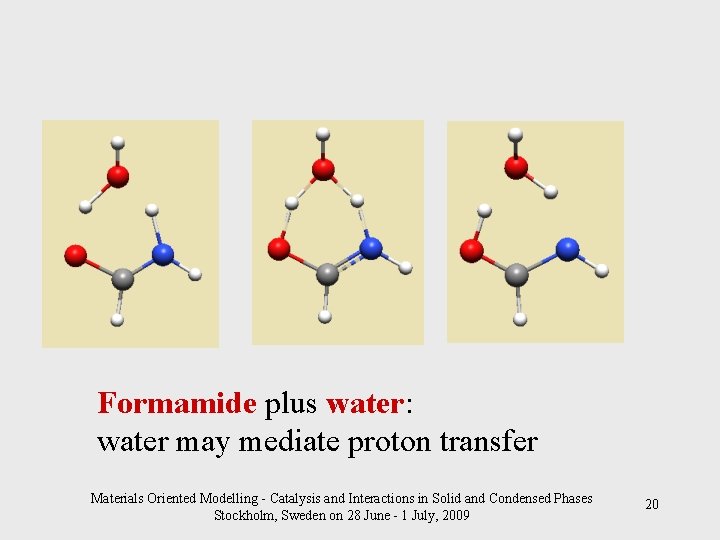
Formamide plus water: water may mediate proton transfer Materials Oriented Modelling - Catalysis and Interactions in Solid and Condensed Phases Stockholm, Sweden on 28 June - 1 July, 2009 20

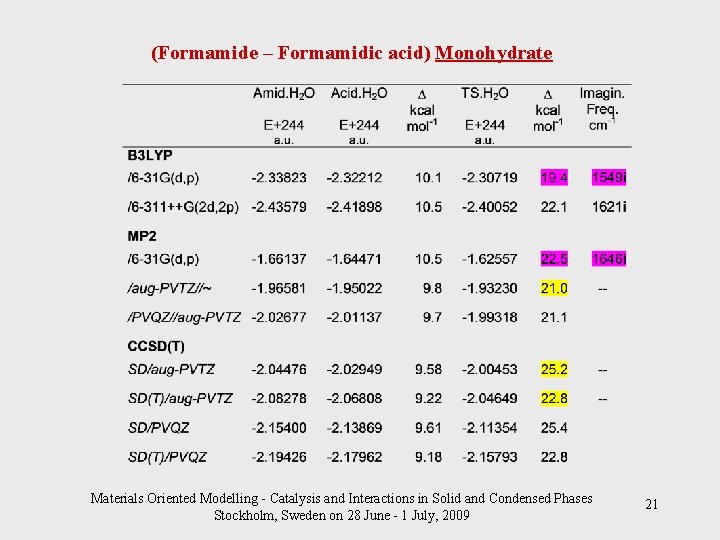
(Formamide – Formamidic acid) Monohydrate Materials Oriented Modelling - Catalysis and Interactions in Solid and Condensed Phases Stockholm, Sweden on 28 June - 1 July, 2009 21

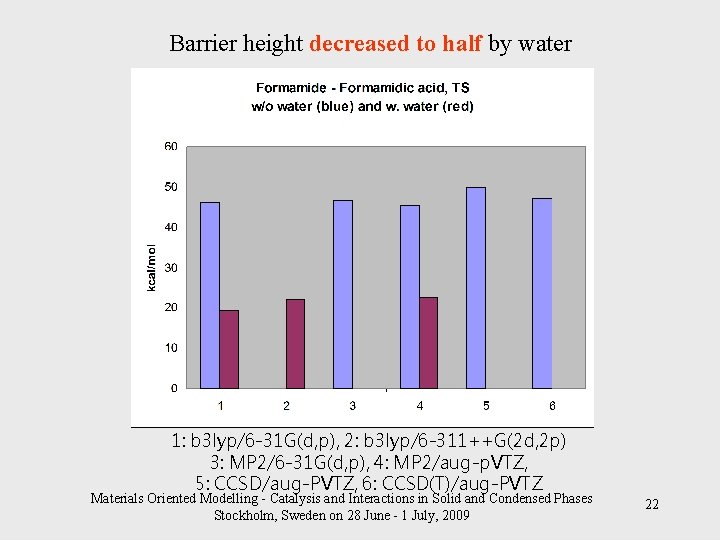
Barrier height decreased to half by water 1: b 3 lyp/6 -31 G(d, p), 2: b 3 lyp/6 -311++G(2 d, 2 p) 3: MP 2/6 -31 G(d, p), 4: MP 2/aug-p. VTZ, 5: CCSD/aug-PVTZ, 6: CCSD(T)/aug-PVTZ Materials Oriented Modelling - Catalysis and Interactions in Solid and Condensed Phases Stockholm, Sweden on 28 June - 1 July, 2009 22

Section 3: Dynamics: Try to ‘see’ the process of watermediated tautomerization Specifically: is there an intermediate state ? Method: ab initio dynamics* Contrary to Car-Parrinello, the wave function is truly recalculated at each point …. * Pulay, P. , Fogarasi, G. : Fock matrix dynamics, CPL 386, 272 -278 (2004) Materials Oriented Modelling - Catalysis and Interactions in Solid and Condensed Phases Stockholm, Sweden on 28 June - 1 July, 2009 23

About ab initio dynamics The notion of reaction mechanisms is based on the Born-Oppenheimer (B-O) approximation: atoms move on a potential energy surface (PES) defined by the electronic energy as a function of nuclear positions. In the simplest models reactions follow the minimum energy pathway (MEP), going through a transition state (TS). The MEP expressed in mass-weighted Cartesians is referred to as the internal reaction coordinate, IRC. Recent computations have shown that reactions may follow a route totally different from the IRC. (W. L. Hase, Science 2002; M. Dupuis, Science 2003). Materials Oriented Modelling - Catalysis and Interactions in Solid and Condensed Phases Stockholm, Sweden on 28 June - 1 July, 2009 24

True dynamics calculations require knowledge of the complete PES, and recent methods generate it "on the fly". The well-known Car. Parrinello method is most efficient computationally because the electronic wave function is "propagated", and not optimized, at the trajectory points. As a consequence, the system is moving close to, but not exactly on the B-O surface. In B-O dynamics, the wave function of a QC method is fully optimized in each step along the trajectory. Energy and first derivatives are determined from ab initio wf, and atomic movements calculated from them classically. This is the approach adopted here. Materials Oriented Modelling - Catalysis and Interactions in Solid and Condensed Phases Stockholm, Sweden on 28 June - 1 July, 2009 25

Dynamics procedure 1. Start geometry not too far from equilibrium 2. The atomic nuclei get a random kick such that the average kinetic energy corresponds to a selected temperature. 3. Energy and FORCES calculated from ab initio Quantum Chemistry ‘on the fly’ 4. Movement of atoms calculated classically 5. GOTO 3 Materials Oriented Modelling - Catalysis and Interactions in Solid and Condensed Phases Stockholm, Sweden on 28 June - 1 July, 2009 26

Actual details: - 1 fs time steps - just 200 steps per trajectory. - but: several hundred trajectories. The QC method: DFT(B 3 LYP)/6 -31 G** or: MP 2/6 -31 G** Materials Oriented Modelling - Catalysis and Interactions in Solid and Condensed Phases Stockholm, Sweden on 28 June - 1 July, 2009 27

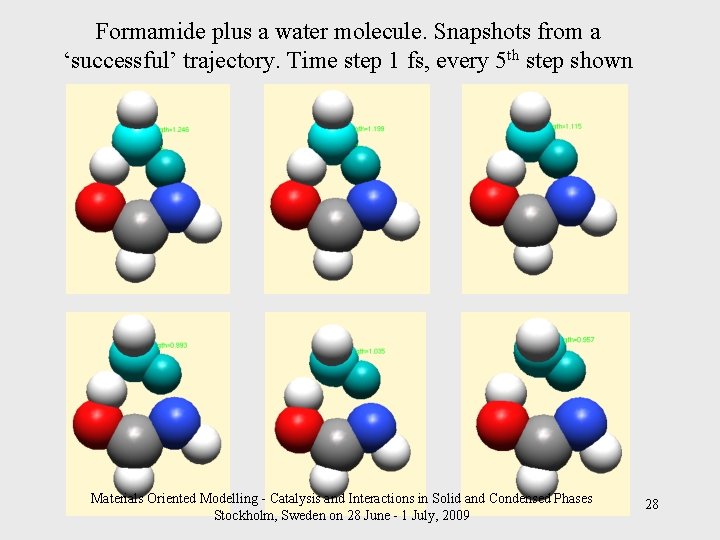
Formamide plus a water molecule. Snapshots from a ‘successful’ trajectory. Time step 1 fs, every 5 th step shown Materials Oriented Modelling - Catalysis and Interactions in Solid and Condensed Phases Stockholm, Sweden on 28 June - 1 July, 2009 28

Formamide dynamics movie: Call PQS Two different cases: 1391 c and 1397 c Materials Oriented Modelling - Catalysis and Interactions in Solid and Condensed Phases Stockholm, Sweden on 28 June - 1 July, 2009 29

Section 4: Back to Cytosine Try to ‘see’ the process of watermediated tautomerization Materials Oriented Modelling - Catalysis and Interactions in Solid and Condensed Phases Stockholm, Sweden on 28 June - 1 July, 2009 30

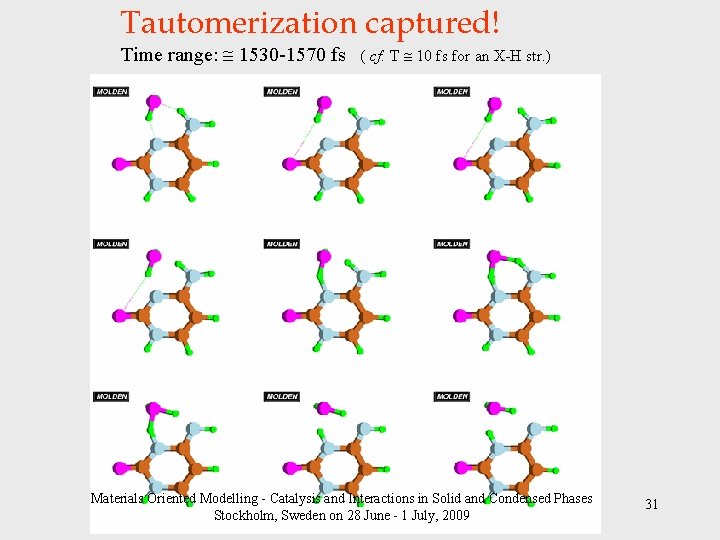
Tautomerization captured! Time range: 1530 -1570 fs ( cf: T 10 fs for an X-H str. ) Materials Oriented Modelling - Catalysis and Interactions in Solid and Condensed Phases Stockholm, Sweden on 28 June - 1 July, 2009 31

Movie. . The End Materials Oriented Modelling - Catalysis and Interactions in Solid and Condensed Phases Stockholm, Sweden on 28 June - 1 July, 2009 32