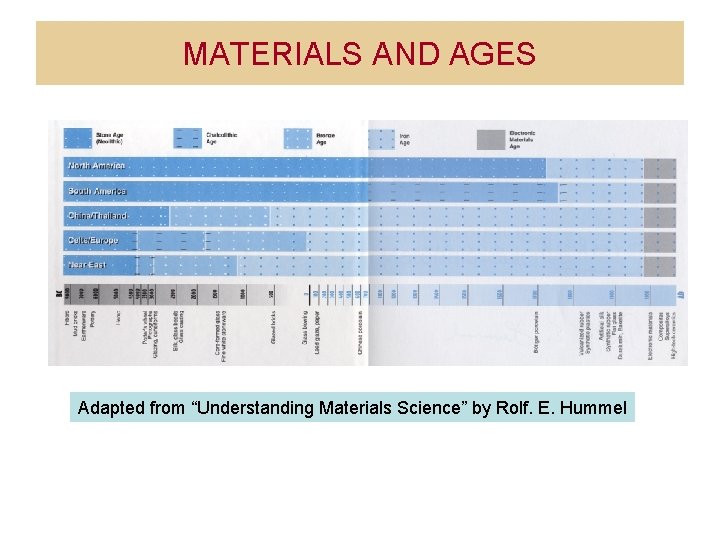
MATERIALS AND AGES Adapted from Understanding Materials Science


- Slides: 10

MATERIALS AND AGES Adapted from “Understanding Materials Science” by Rolf. E. Hummel

Polymeric Materials Adapted from “Understanding Materials Science” by Rolf. E. Hummel Ø Fibers ØWool (2 million years) ØHemp (First cultivated plant, 6500 years) ØFlax (5400 years - Eygypt) ØCotton (5000 years - India) ØSilk (5000 -4500 years – China and Japan) Ø Artificial Silk (1880’s – regenerated cellulose (a complex carbohydrate) fibers later known as Viscose or Rayon) Ø Rubber Ø Mayan Culture Ø Brought to Europe in 1496 Ø The “milk” (Latex) used for water proofing in 1615. Ø Rubber items such as air mattresses, potable bath tubs and “mackintoshes” in 1800’s. Ø Charles Goodyear in 1830’s discovered ‘Vulcanization” – treating with sulfur and curing rubber to make it durable and pliable at low temperatures. Ø Wood, Leather, Cork, Sponges, etc.

Polymeric Materials Adapted from “Understanding Materials Science” by Rolf. E. Hummel Ø Paper-making: China, A. D. 105 Ø Plastics Ø Schönbein (Germany, 1846): Nitrocellulose or cellulose nitrate – a Thermoplastic material, i. e. it will become pliable with heating. Ø L. H. Baekeland (Amero-Belgian, 1906): Bakelite – the first fully synthetic organic polymer which is a Thermoset plastic, i. e. it remains hard at elevated temperatures. Ø Goodyear (USA, 1842): Vulcanized rubber – an Elastomer.

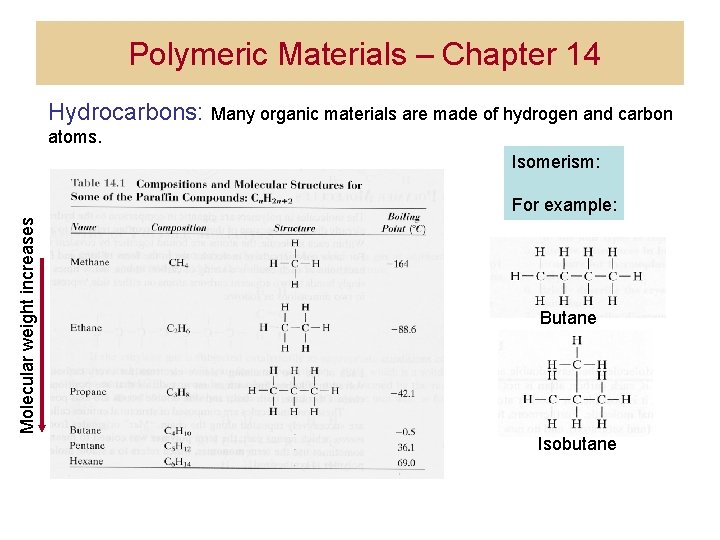
Polymeric Materials – Chapter 14 Hydrocarbons: Many organic materials are made of hydrogen and carbon atoms. Isomerism: Molecular weight increases For example: Butane Isobutane

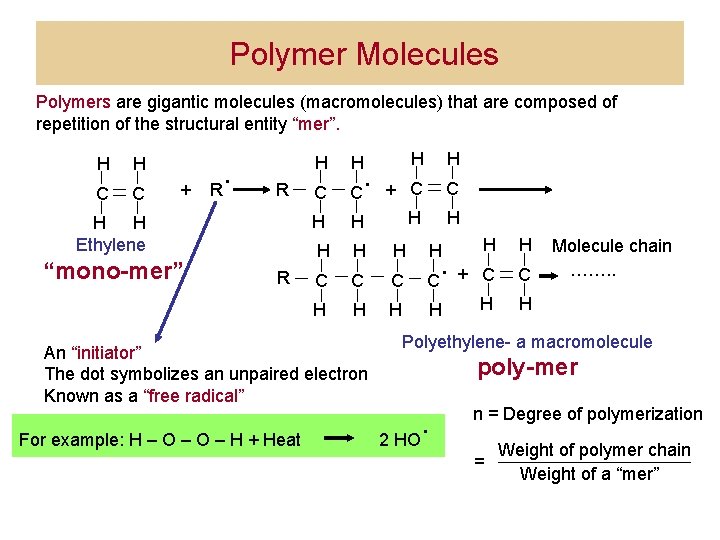
Polymer Molecules Polymers are gigantic molecules (macromolecules) that are composed of repetition of the structural entity “mer”. H C + R . R H H Ethylene “mono-mer” R H H C C + C C H H H H H C C H H H C + C H H . An “initiator” The dot symbolizes an unpaired electron Known as a “free radical” For example: H – O – H + Heat . Molecule chain ……. . H Polyethylene- a macromolecule poly-mer 2 HO . n = Degree of polymerization = Weight of polymer chain Weight of a “mer”

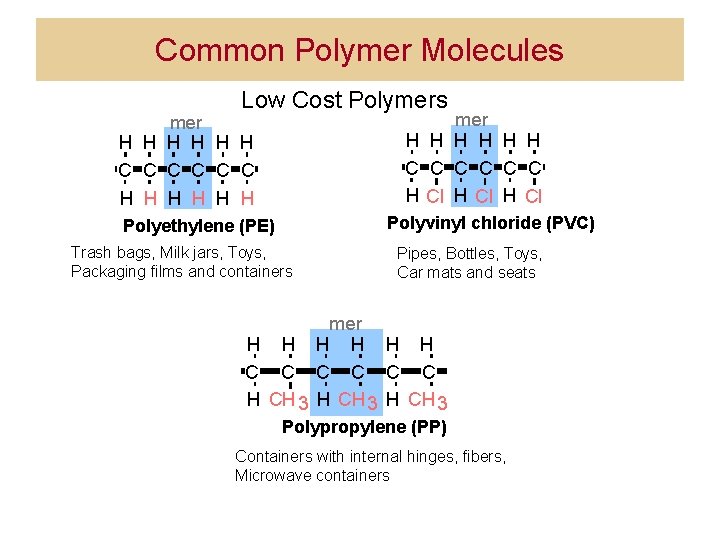
Common Polymer Molecules Low Cost Polymers mer H H H C C C H Cl mer H H H C C C H H H Polyvinyl chloride (PVC) Polyethylene (PE) Trash bags, Milk jars, Toys, Packaging films and containers Pipes, Bottles, Toys, Car mats and seats mer H H H C C C H CH 3 Polypropylene (PP) Containers with internal hinges, fibers, Microwave containers

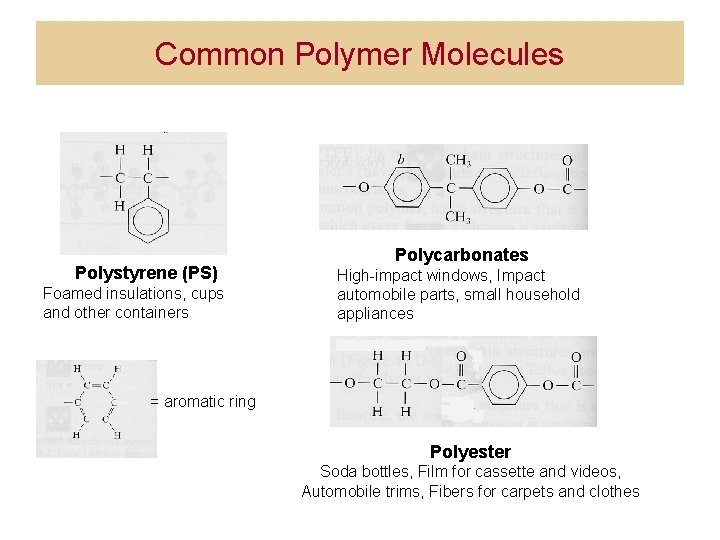
Common Polymer Molecules Polystyrene (PS) Foamed insulations, cups and other containers Polycarbonates High-impact windows, Impact automobile parts, small household appliances = aromatic ring Polyester Soda bottles, Film for cassette and videos, Automobile trims, Fibers for carpets and clothes

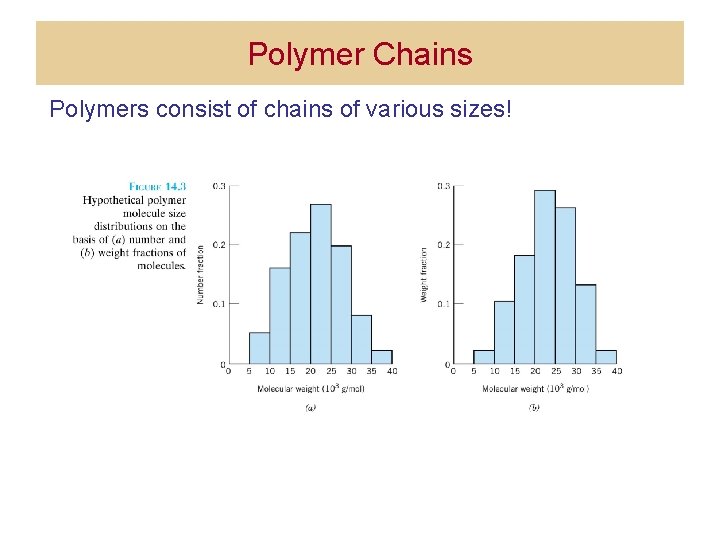
Polymer Chains Polymers consist of chains of various sizes!

Average Molecular Weight Number Average: Mn = (Mi)(xi), Mi = mean molecular weight of the size range “i”, xi = fraction of the chains within the size range “i”. nn = Degree of polymerization = Mn / m Experimentally the Mn is calculated by knowing the total weight of a polymer (Mtotal) in a solution and measuruing the number of chain ends (nend): Mn = 2 Mtotal / nend The size distribution is evaluated by Size Exclusion Chromotography (SEC) Weight Average: MW = (Mi)(wi), wi = weight fraction of the chains within the range “i”. nw = Degree of polymerization = Mw / m See Example 14. 1 in your book!

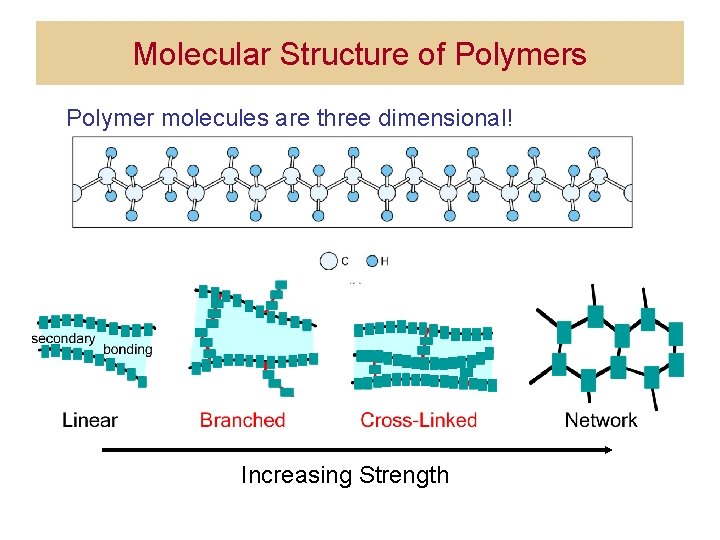
Molecular Structure of Polymers Polymer molecules are three dimensional! Increasing Strength