Material World Section 2 Transformation of Matter Physical





- Slides: 8

Material World Section 2: Transformation of Matter

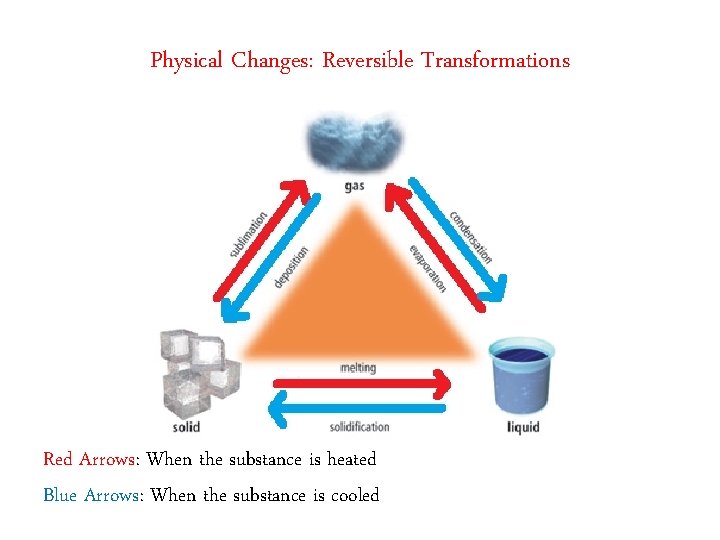
Physical Changes Physical Change: When the appearance of a substance changes but the nature of the substance remains the same. Example: ice melting to liquid water, cutting a paper àNo new substances are created. àThe characteristic properties of the substance are preserved. àChanges in states of matter are physical changes. àPhysical Changes are reversible.

Physical Changes: Reversible Transformations Red Arrows: When the substance is heated Blue Arrows: When the substance is cooled

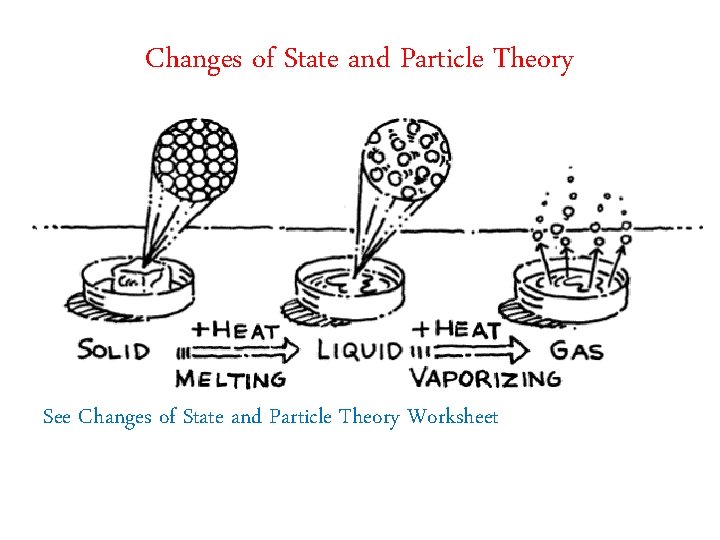
Changes of State and Particle Theory See Changes of State and Particle Theory Worksheet

Try This! 1. a) Name three states of matter. b) Name the six change in states of matter. c) Give an example of each of these changes in states of matter. 2. What is a physical change? 3. Are new substances produced during a physical change? Explain.

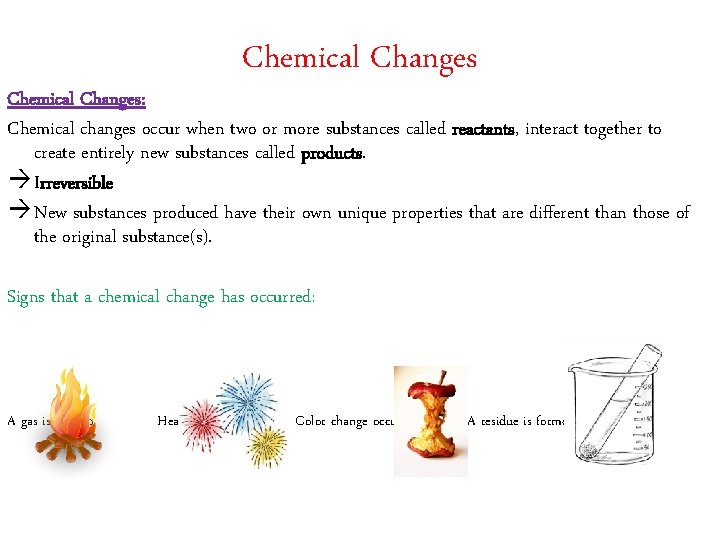
Chemical Changes: Chemical changes occur when two or more substances called reactants, interact together to create entirely new substances called products. à Irreversible à New substances produced have their own unique properties that are different than those of the original substance(s). Signs that a chemical change has occurred: A gas is formed Heat/light is produced Color change occurs A residue is formed

Try This! 1. What is the difference between a chemical change and a physical change? Give some examples for each. 2. Are new substances produced during a chemical change? 3. What happens to the characteristic properties of a substance when a chemical change occurs? 4. What four signs indicate a chemical change?

Try This! 6. Indicate which of the statements below describe a physical change and which describe a chemical change: a) b) c) d) e) Sugar dissolves in water. The filament in a light bulb glows when an electric current runs through it. A piece of chalk is crushed. Snow melts. Lime water (clear in color) becomes cloudy in color in the presence of carbon dioxide.