Material Science Properties of Materials 1 Introduction n























- Slides: 28

Material Science Properties of Materials 1

Introduction n n The success and expertise of the design engineer will be partly based on his or her knowledge of materials and how they flow or flow during the manufacturing process Materials selection for a product is based upon a consideration of the properties required. These include: n n n Mechanical properties Physical properties Chemical properties 2

Physical Properties n n n Physical properties are the properties inherit in the material due to the chemical and physical structure of the matter in the material. Those properties or behaviour in connection with heat, sound, light, electricity, and magnetic environment These include density, melting point, boiling point, thermal conductivity, coefficient of expansion, electric conductivity, electric insulation 3

Density PHYSICAL PROPERTIES n Density of a material is defined as its mass per unit volume n The SI unit for density is kg m-3 n Density of water = 1, 000 kg m-3 n Specific gravity of a material is the ratio of the density of the material to that of water n Wood ( 0. 8 ) , aluminium ( 2. 7 ), copper ( 8. 9 ), lead ( 11. 3) Iron ( 7. 8 ) 4

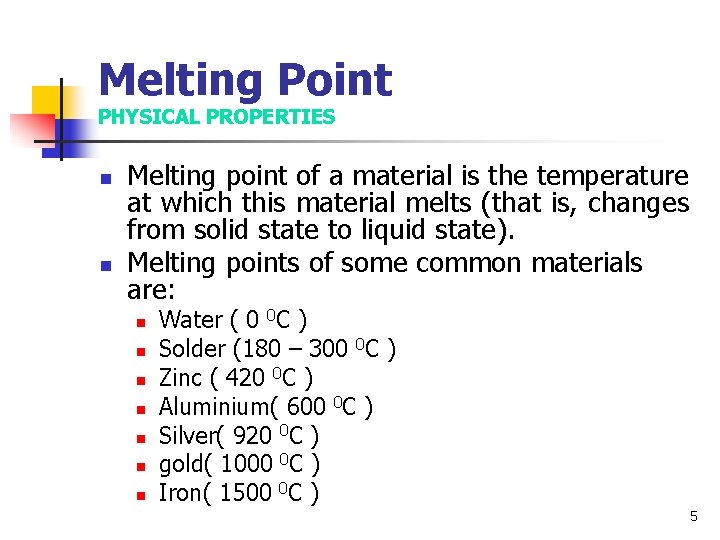
Melting Point PHYSICAL PROPERTIES n n Melting point of a material is the temperature at which this material melts (that is, changes from solid state to liquid state). Melting points of some common materials are: n n n n Water ( 0 0 C ) Solder (180 – 300 0 C ) Zinc ( 420 0 C ) Aluminium( 600 0 C ) Silver( 920 0 C ) gold( 1000 0 C ) Iron( 1500 0 C ) 5

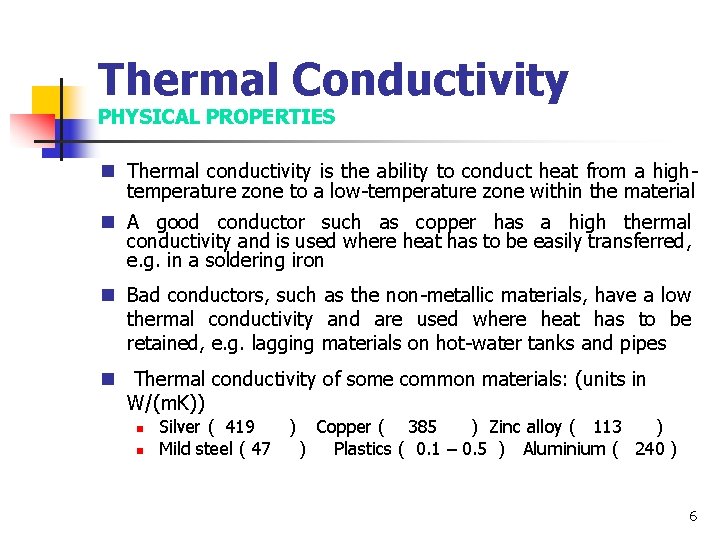
Thermal Conductivity PHYSICAL PROPERTIES n Thermal conductivity is the ability to conduct heat from a hightemperature zone to a low-temperature zone within the material n A good conductor such as copper has a high thermal conductivity and is used where heat has to be easily transferred, e. g. in a soldering iron n Bad conductors, such as the non-metallic materials, have a low thermal conductivity and are used where heat has to be retained, e. g. lagging materials on hot-water tanks and pipes n Thermal conductivity of some common materials: (units in W/(m. K)) n n Silver ( 419 ) Copper ( 385 ) Zinc alloy ( 113 ) Mild steel ( 47 ) Plastics ( 0. 1 – 0. 5 ) Aluminium ( 240 ) 6

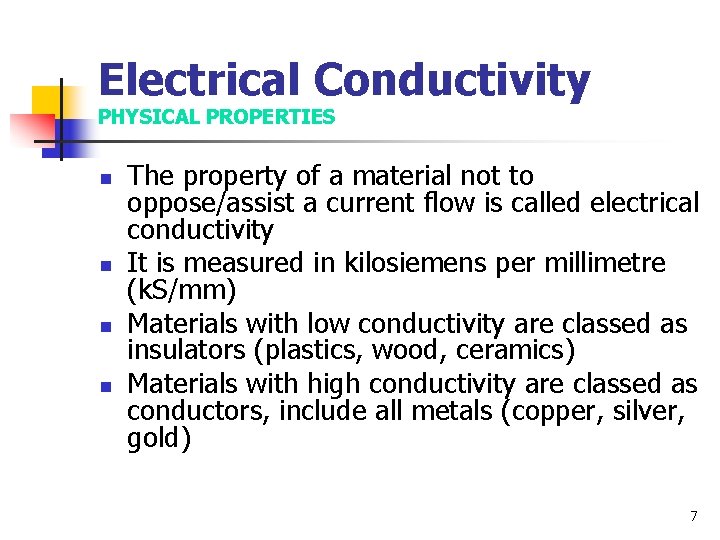
Electrical Conductivity PHYSICAL PROPERTIES n n The property of a material not to oppose/assist a current flow is called electrical conductivity It is measured in kilosiemens per millimetre (k. S/mm) Materials with low conductivity are classed as insulators (plastics, wood, ceramics) Materials with high conductivity are classed as conductors, include all metals (copper, silver, gold) 7

Mechanical Properties n n Mechanical properties are the properties produced by the material whilst being subjected to various load conditions. This properties are very important to engineer They influence both the design and choice of the production procedure Mechanical properties include: 1. Ductility 2. Malleabilit y 3. Toughness 4. Strength 5. Brittleness 6. Hardness 7. Elasticity 8

Ductility MECHANICAL PROPERTIES n n n A ductility material has the ability to ‘flow’ and be drawn out when subjected to tensile loading This means that the material can under go plastic deformation without fracture Ductility determines the amount that a metal can be cold-worked without fracture If the metal is heated the ductility is reduced due to the lowering of material strength A typical example of a manufacturing process which is dependent upon the ductility is the drawing of wire 9

Malleability MECHANICAL PROPERTIES n n A malleability material has the ability to withstand plastic deformation when subject to compressive force Malleability materials tend to be weak in tension and may be tear when loaded The malleability of a material is normally increased when it is subjected to high temperature Rolling, forging and extruding are examples of the processes requiring malleability 10

Toughness MECHANICAL PROPERTIES n n This property enable a material to withstand bending , forming and stock loading without fracture All materials contain cracks, many of them are invisible to the naked eye The forces that applied to the materials tend to increase the size of these cracks until a fracture occurs Toughness is the resistance of the material to growth of these cracks, the more resistance, the tougher is the material 11

Strength MECHANICAL PROPERTIES n n n The strength of a material can be defined as the ‘ability to withstand the application of forces without fracture’ Tensile, compressive and shear strength are common forms of strength The different strengths are related to the type of forces applied to the material The strength most commonly used is tensile Tensile strength is measured as the max. load required to fracture unit area of a material The unit of strength is MN/m 2 12

Brittleness MECHANICAL PROPERTIES n n n Brittleness can be thought of as opposite of toughness A brittle material offers little resistance to the growth of cracks, and the crack grows rapidly through the material Brittle materials break without appreciable deformation and at low stress, glass is a good example 13

Hardness MECHANICAL PROPERTIES n n n Hardness of a material is the resistance that the material offers to deformation or scratching The smaller the deformation or the more difficult to mark the surface by a scratch, then the harder is the material There are 2 primary form of testing: n n Indentation of material by either a hardened steel ball or a diamond Scratching the surface of the material 14

Elasticity MECHANICAL PROPERTIES n n n The elasticity of a material can be said to be the resistance offered by the material to elastic or temporary deformation when subject to forces The material deforms due to the applied forces, but return to its original shape and size when the forces are removed The measure of elasticity is given the name ‘Young’s modulus of elasticity’, and is a constant for each material 15

Chemical Properties n n These are relevant in considerations of corrosion and solvent resistance Materials that do not chemically break down in an alien environment are considered to have good corrosion resistant qualities The ‘alien’ environment may be salt water moist air, polluted atmosphere or highly acidic atmosphere or solutions Other properties such as fire resistant and temperature resistant will also be applicable in some situation 16

Corrosion 17

Corrosion n Corrosion can be defined as the unintended destructive chemical or electrochemical reaction of a material with its environment. Metals, polymers, and ceramics are susceptible to attack from different environments. The corrosion of metals is mainly electrochemical in nature, whereas the other materials usually involve chemical reaction. 18

The Corrosion of Metals I n n n All metals corrode in a greater or lesser extent. Even precious metals like gold and silver tarnish in time which is also a form of corrosion. Corrosion prevention processes are unable to prevent the failure of the component by corrosion, but they can only slow down the corrosion process. Corrosion is the slow but continuous eating away of metallic components by chemical or electrochemical attack. 19

The Corrosion of Metals II n There are four major ways in which metals corrode : n n Dry Corrosion Wet Corrosion Galvanic Corrosion Atmospheric Corrosion 20

Dry corrosion n Dry corrosion is the direct oxidation of metals It occurs when a freshly cut surface reacts with the oxygen in the atmosphere Corrosion resistant metals, such as lead, zinc and aluminum can form a dry oxide film on the surface which protects the metals from further atmospheric attack 21

Wet corrosion occurs in two ways : n The oxidation of metals in the presence of air and moisture, such as the rusting of ferrous (iron-based) metals. n The corrosion of metals by reaction with the dilute acids in rain water (acid rain) 22

Galvanic corrosion n n Galvanic corrosion occurs when two dissimilar metals are in intimate contact, such as iron and zinc. In galvanic corrosion, one of the metals with higher electro-negativity will be corroded while the other is protected. 23

Atmospheric Corrosion n n Any metal when exposed to normal atmospheric conditions will be covered with an invisible, thin film of moisture This moisture film will be contaminated with dissolved solids and gases, thus increase the rate of corrosion The most common example of corrosion due to dissolved oxygen from the atmosphere is the formation of “red rust” on unprotected ferrous metals. The major cost of corrosion prevention is largely related to atmospheric corrosion. 24

Factors Affecting Corrosion n Structural design : e. g. crevices and corners can easily trap n Environment : e. g. metals in acidic environment will corrode n Composition of structure : e. g. the presence of impurities in n Temperature : corrosion can be sped up badly in hot and moisture that will increase the rate of corrosion; and the inappropriate selection of materials for the components rapidly non-ferrous metals reduces their corrosion resistance; whereas the inclusion of certain alloying elements such as nickel and chromium can improve the corrosion resistance of alloys e. g. stainless steel humid condition. 25

Corrosion Prevention I 1. The Use of Corrosion Resistant Materials n Some metals can react with oxygen to form a passive layer on their surfaces which prevent the metal inside from further corrosion. n Aluminum alloys, copper alloys and stainless steels are typical examples of this category. n These metals are suitable for use in corrosive environment. 26

Corrosion Prevention II 2. Protective Coatings n n Protective coatings on the surface of metals can also improve the corrosion resistance of metals. Hot dipping, electroplating, anodizing, plastic coatings, and painting are commonly used coating methods. 27

Corrosion Prevention III 3. Cathodic Protection n This can be applied to most metals. For example, the buried iron pipes can be protected from corrosion by applying a negative voltage onto them with respect to the surrounding soil. n Also, cathodic protection can be applied to protect the steel hulls of ships. 28