Material Balances w Chemical Reactions Section 4 6Chemical

Material Balances w/ Chemical Reactions
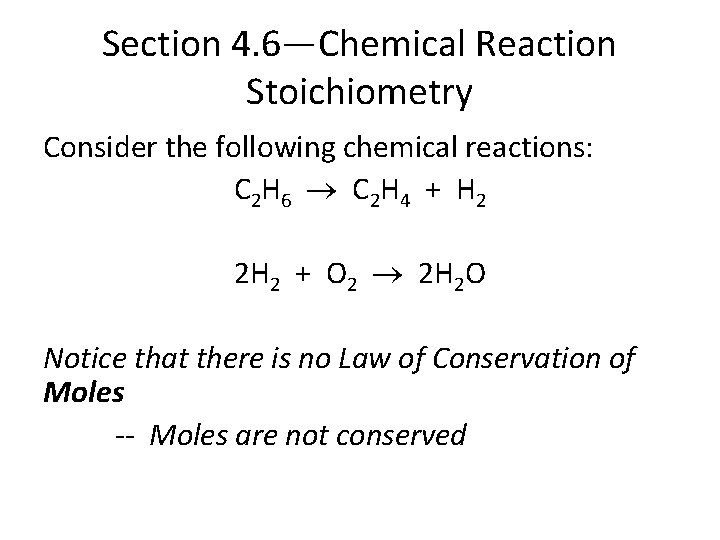
Section 4. 6—Chemical Reaction Stoichiometry Consider the following chemical reactions: C 2 H 6 C 2 H 4 + H 2 2 H 2 + O 2 2 H 2 O Notice that there is no Law of Conservation of Moles -- Moles are not conserved
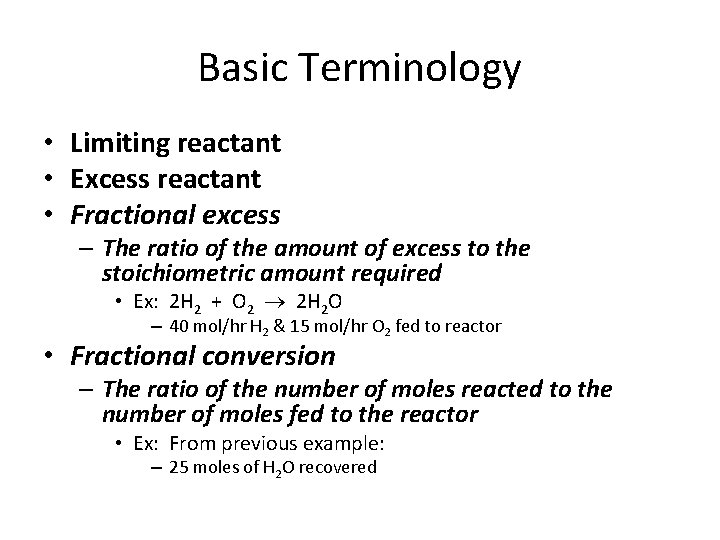
Basic Terminology • Limiting reactant • Excess reactant • Fractional excess – The ratio of the amount of excess to the stoichiometric amount required • Ex: 2 H 2 + O 2 2 H 2 O – 40 mol/hr H 2 & 15 mol/hr O 2 fed to reactor • Fractional conversion – The ratio of the number of moles reacted to the number of moles fed to the reactor • Ex: From previous example: – 25 moles of H 2 O recovered
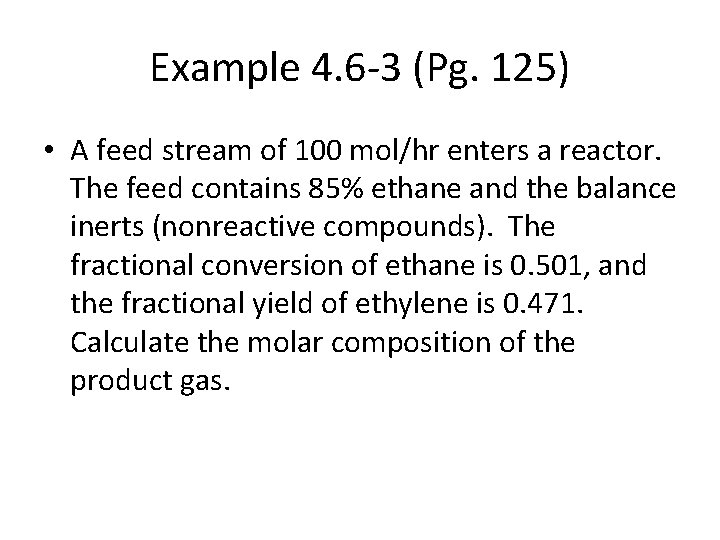
Example 4. 6 -3 (Pg. 125) • A feed stream of 100 mol/hr enters a reactor. The feed contains 85% ethane and the balance inerts (nonreactive compounds). The fractional conversion of ethane is 0. 501, and the fractional yield of ethylene is 0. 471. Calculate the molar composition of the product gas.
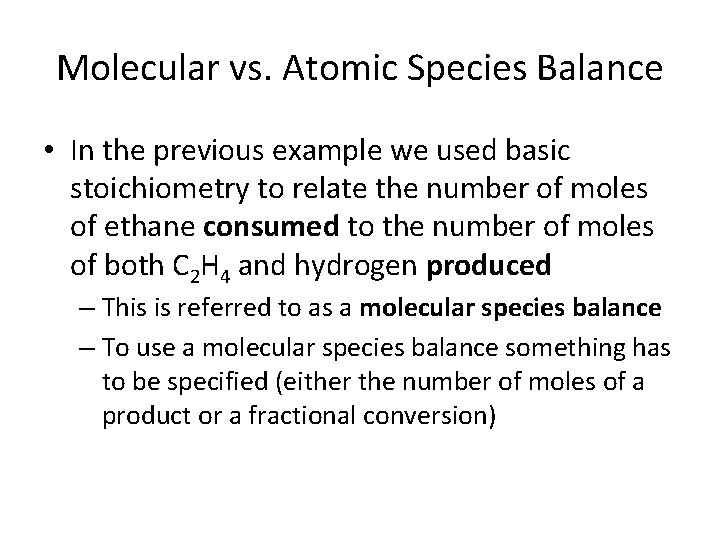
Molecular vs. Atomic Species Balance • In the previous example we used basic stoichiometry to relate the number of moles of ethane consumed to the number of moles of both C 2 H 4 and hydrogen produced – This is referred to as a molecular species balance – To use a molecular species balance something has to be specified (either the number of moles of a product or a fractional conversion)
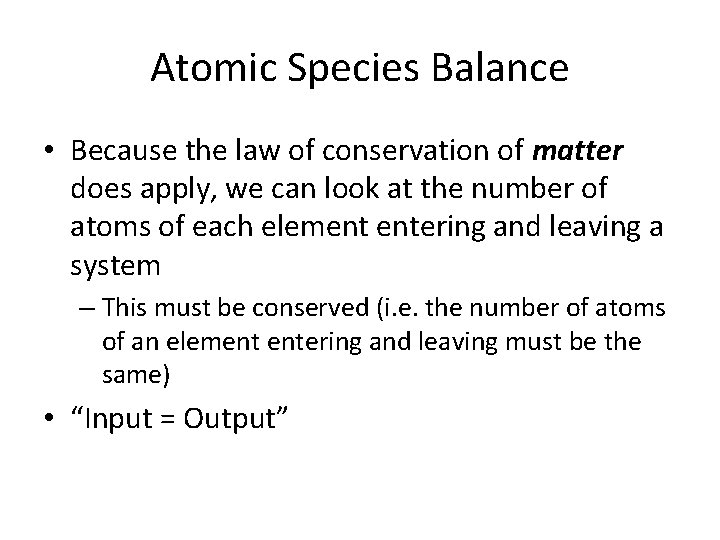
Atomic Species Balance • Because the law of conservation of matter does apply, we can look at the number of atoms of each element entering and leaving a system – This must be conserved (i. e. the number of atoms of an element entering and leaving must be the same) • “Input = Output”
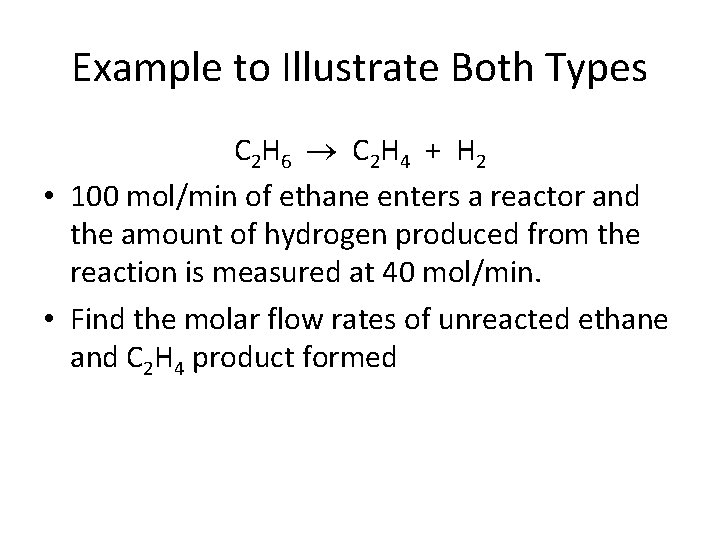
Example to Illustrate Both Types C 2 H 6 C 2 H 4 + H 2 • 100 mol/min of ethane enters a reactor and the amount of hydrogen produced from the reaction is measured at 40 mol/min. • Find the molar flow rates of unreacted ethane and C 2 H 4 product formed
- Slides: 7