MATERIAL BALANCES SEPARATIONS CH EN 170 UNITS MIXTURE

MATERIAL BALANCES: SEPARATIONS CH EN: 170
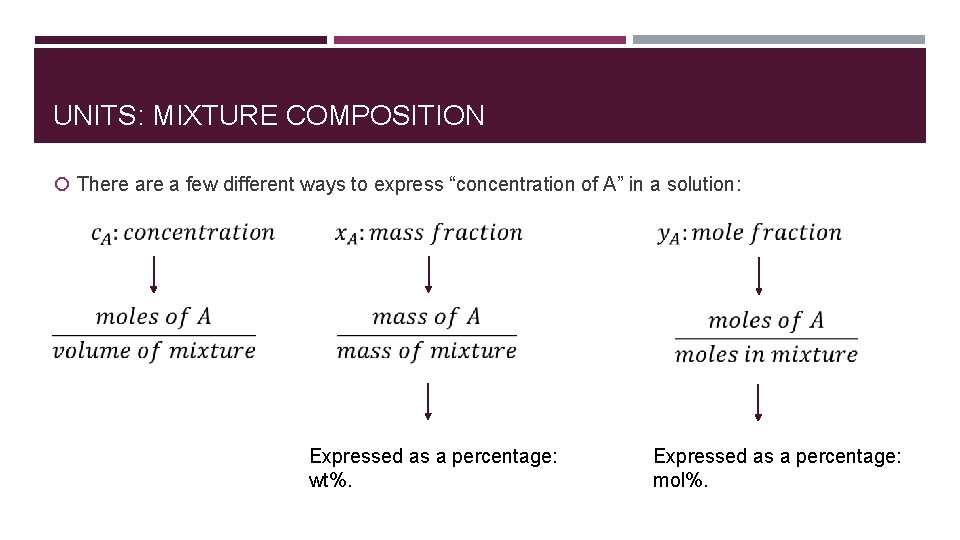
UNITS: MIXTURE COMPOSITION There a few different ways to express “concentration of A” in a solution: Expressed as a percentage: wt%. Expressed as a percentage: mol%.
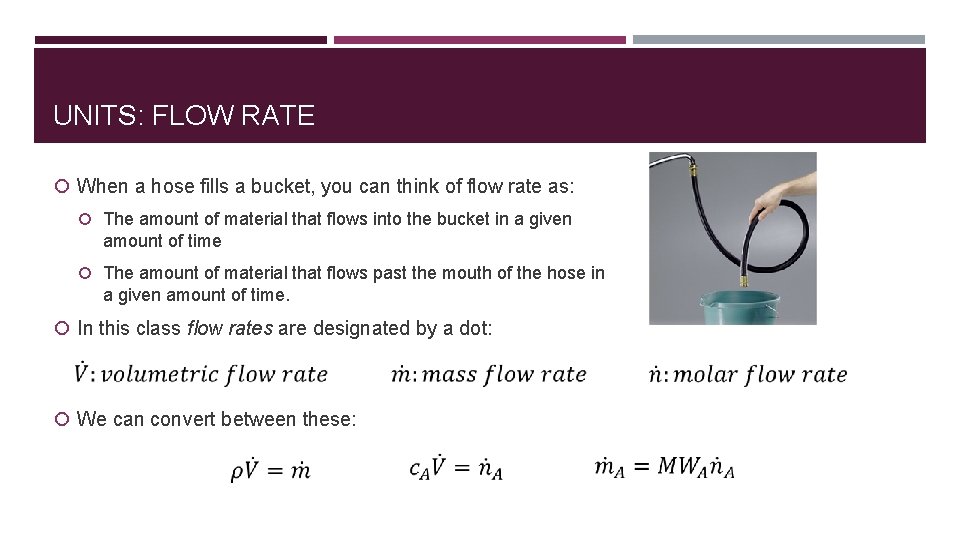
UNITS: FLOW RATE When a hose fills a bucket, you can think of flow rate as: The amount of material that flows into the bucket in a given amount of time The amount of material that flows past the mouth of the hose in a given amount of time. In this class flow rates are designated by a dot: We can convert between these:

PRACTICE: COMPOSITION AND FLOW RATE Consider two chemicals, A and B. MWA = 2 gm/mol; MWB = 5 gm/mol 0. 1 M (gmol/L) solution of A in water flows at 1000 L/hr. What is the mass flow rate of A in kg/hr? Equimolar mixture of A and B flows at a total molar flowrate of 100 lbmol/min. What is the total mass flow rate in lbm/min? 20 wt% mixture of A and B has a density of 10 kg/m 3. Mass flow rate of A is 10 kg/min. What is the volumetric flowrate of the mixture in m 3/min?

MATERIAL BALANCES Balances are powerful tools for understanding the world and solving problems. A material balance is a particular way of viewing/utilizing the conservation of mass. Consider a system with mass flowing in and out. We can do a balance over this system: Rate of mass flowing in. Rate of mass flowing out. Rate of accumulation of mass.

STEADY STATE MASS BALANCE What happens to this formula when a system is steady-state? Rate of mass flowing in. Rate of mass flowing out. Rate of accumulation of mass. Which we can write as: This relationship is a tool we can use to solve problems.
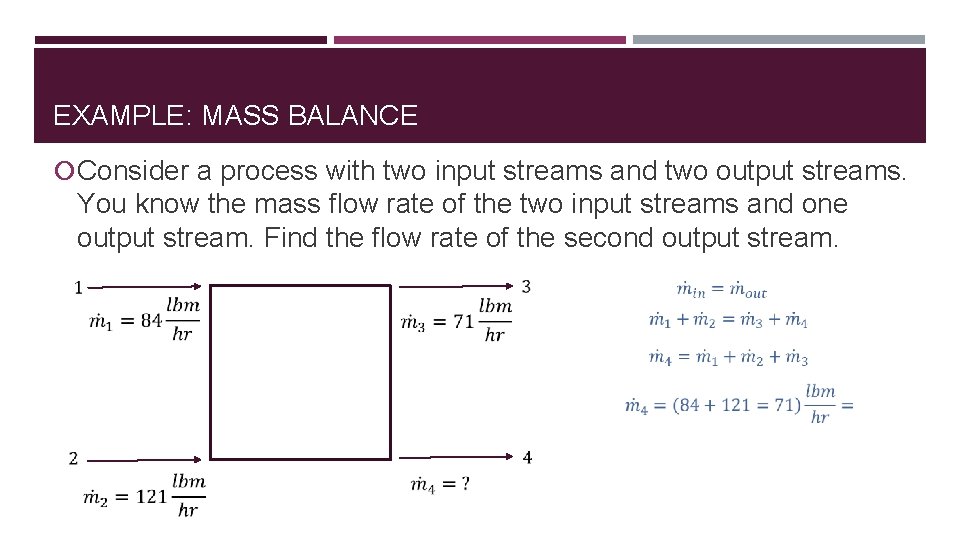
EXAMPLE: MASS BALANCE Consider a process with two input streams and two output streams. You know the mass flow rate of the two input streams and one output stream. Find the flow rate of the second output stream.
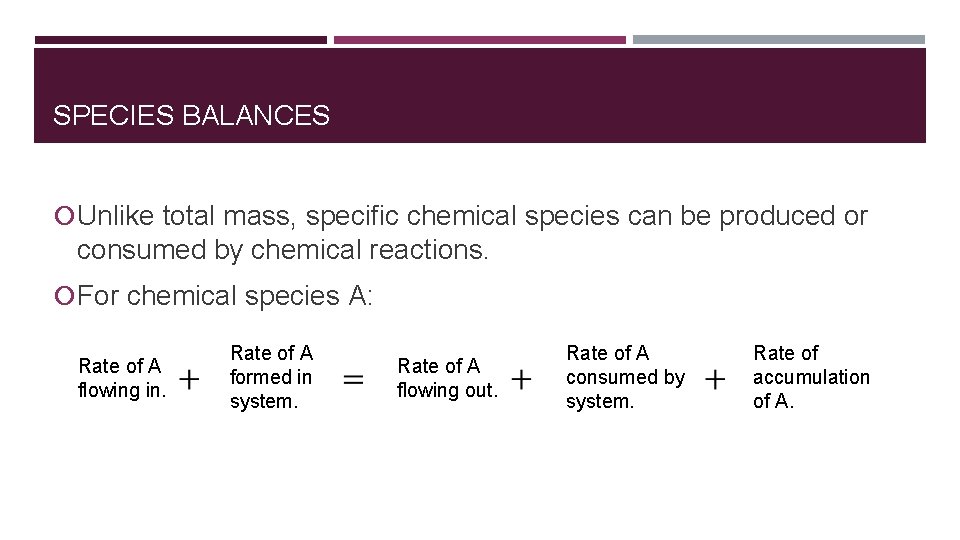
SPECIES BALANCES Unlike total mass, specific chemical species can be produced or consumed by chemical reactions. For chemical species A: Rate of A flowing in. Rate of A formed in system. Rate of A flowing out. Rate of A consumed by system. Rate of accumulation of A.
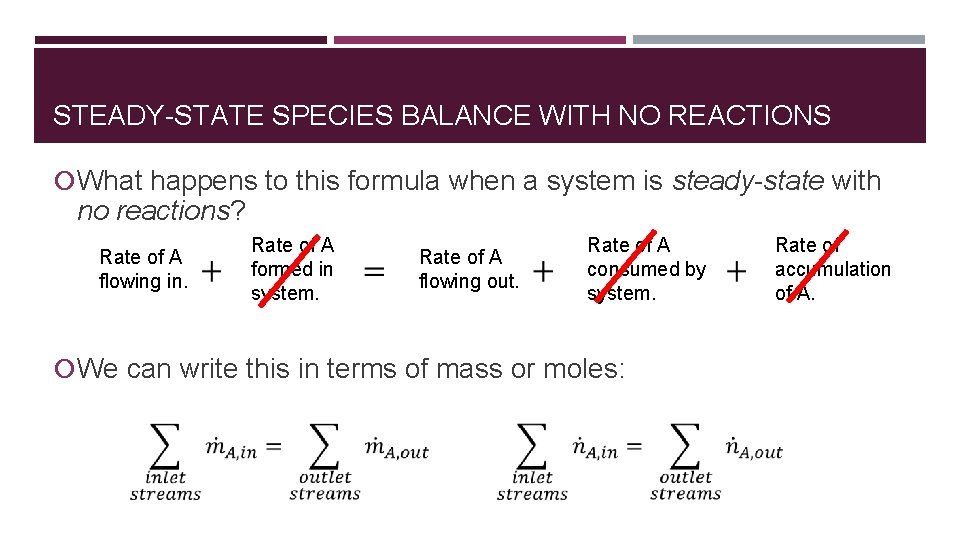
STEADY-STATE SPECIES BALANCE WITH NO REACTIONS What happens to this formula when a system is steady-state with no reactions? Rate of A flowing in. Rate of A formed in system. Rate of A flowing out. Rate of A consumed by system. We can write this in terms of mass or moles: Rate of accumulation of A.
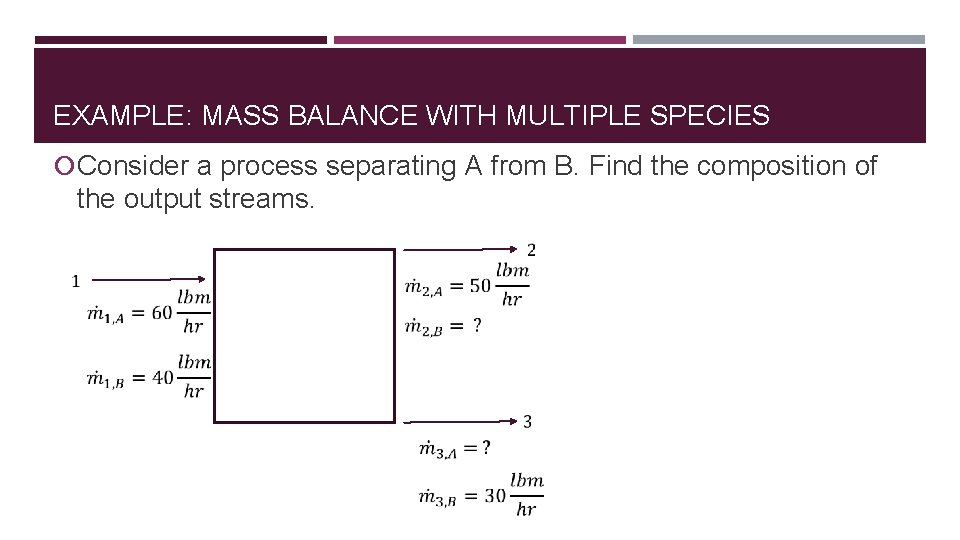
EXAMPLE: MASS BALANCE WITH MULTIPLE SPECIES Consider a process separating A from B. Find the composition of the output streams.

SYSTEMS OF EQUATIONS To find multiple unknowns, we need to solve multiple simultaneous equations (one equation for each unknown) We can get one equation from doing a total mass balance. We can get one equation from each chemical species by doing a species balance The equations need to be independent (no equation is just a rearrangement of another one).
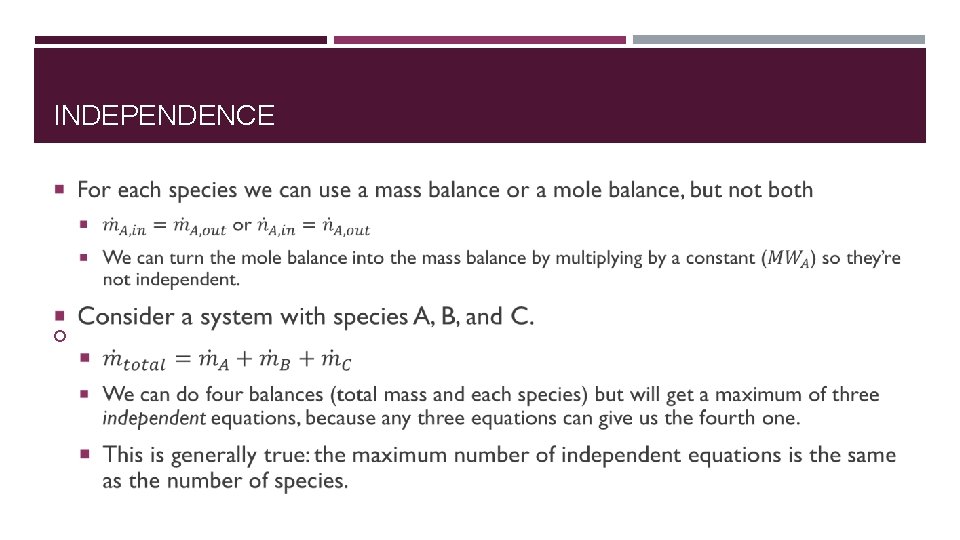
INDEPENDENCE

EXAMPLE: SEPARATION One method of producing drinkable water is to force seawater through a membrane that does not allow the salt to pass through. A large facility continuously filters 2300 L of seawater per hour. The seawater contains sodium chloride (Na. Cl) at a concentration of 1. 4 M. Two streams emerge from the facility: (1) a “brine” stream with a sodium chloride concentration of 5. 6 M and flow rate of 560 L/hr and (2) a drinking water stream. The densities of the three streams can be considered to be the same. What is the sodium chloride concentration in the drinking water.
- Slides: 14