Match the terms to their definitions What do








- Slides: 22

Match the terms to their definitions. What do you notice about some of the definitions?

Determining enthalpy changes 15 October 2021

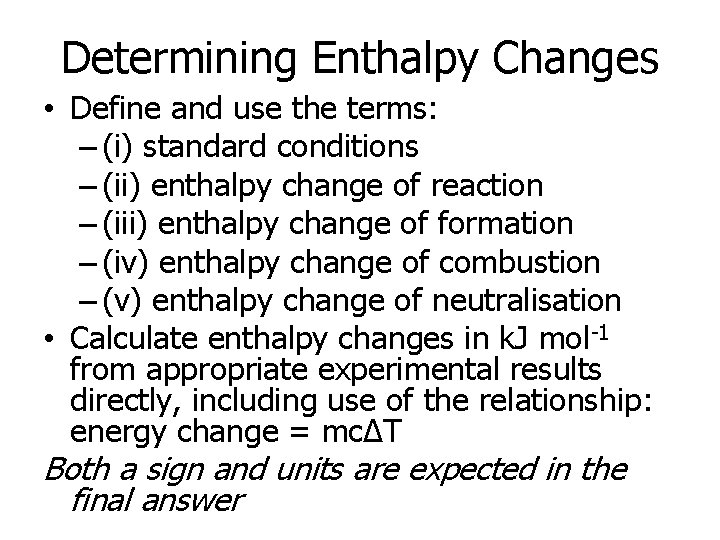
Determining Enthalpy Changes • Define and use the terms: – (i) standard conditions – (ii) enthalpy change of reaction – (iii) enthalpy change of formation – (iv) enthalpy change of combustion – (v) enthalpy change of neutralisation • Calculate enthalpy changes in k. J mol-1 from appropriate experimental results directly, including use of the relationship: energy change = mcΔT Both a sign and units are expected in the final answer

Definitions You will need to learn definitions of: • Standard conditions • Enthalpy change of reaction • Enthalpy change of formation • Enthalpy change of combustion • Enthalpy of neutralisation

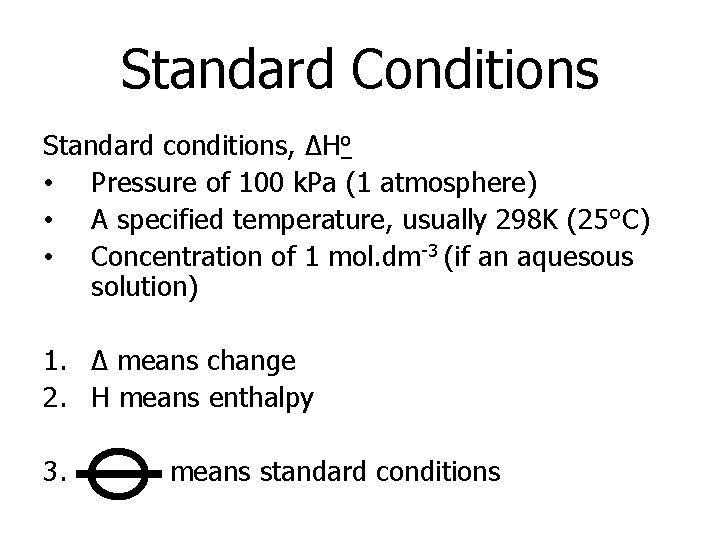
Standard Conditions Standard conditions, ΔHo • Pressure of 100 k. Pa (1 atmosphere) • A specified temperature, usually 298 K (25°C) • Concentration of 1 mol. dm-3 (if an aquesous solution) 1. Δ means change 2. H means enthalpy 3. means standard conditions

Standard Conditions If we want a standard enthalpy change, the substance must be in it’s standard state. This is its natural physical state under standard conditions (at 100 k. Pa and the stated temperature). e. g. 1. Magnesium 2. Hydrogen 3. Water

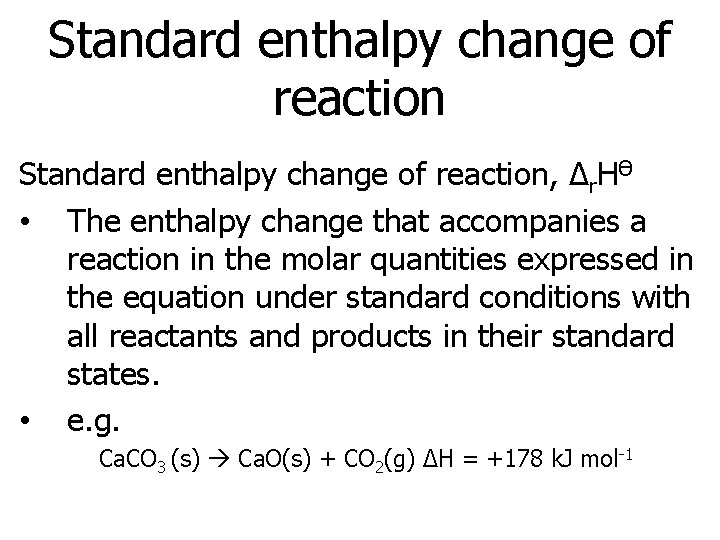
Standard enthalpy change of reaction, Δr. HӨ • The enthalpy change that accompanies a reaction in the molar quantities expressed in the equation under standard conditions with all reactants and products in their standard states. • e. g. Ca. CO 3 (s) Ca. O(s) + CO 2(g) ΔH = +178 k. J mol-1

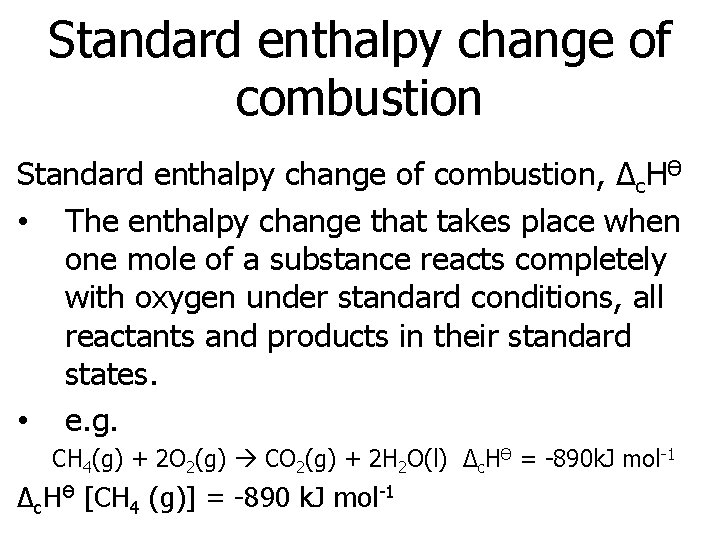
Standard enthalpy change of combustion, Δc. HӨ • The enthalpy change that takes place when one mole of a substance reacts completely with oxygen under standard conditions, all reactants and products in their standard states. • e. g. CH 4(g) + 2 O 2(g) CO 2(g) + 2 H 2 O(l) Δc. HӨ = -890 k. J mol-1 Δc. HӨ [CH 4 (g)] = -890 k. J mol-1

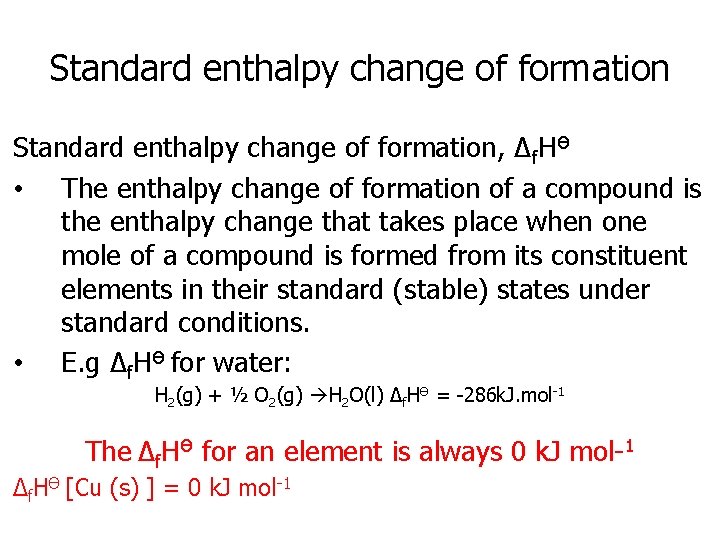
Standard enthalpy change of formation, Δf. HӨ • The enthalpy change of formation of a compound is the enthalpy change that takes place when one mole of a compound is formed from its constituent elements in their standard (stable) states under standard conditions. • E. g Δf. HӨ for water: H 2(g) + ½ O 2(g) H 2 O(l) Δf. HӨ = -286 k. J. mol-1 The Δf. HӨ for an element is always 0 k. J mol-1 Δf. HӨ [Cu (s) ] = 0 k. J mol-1

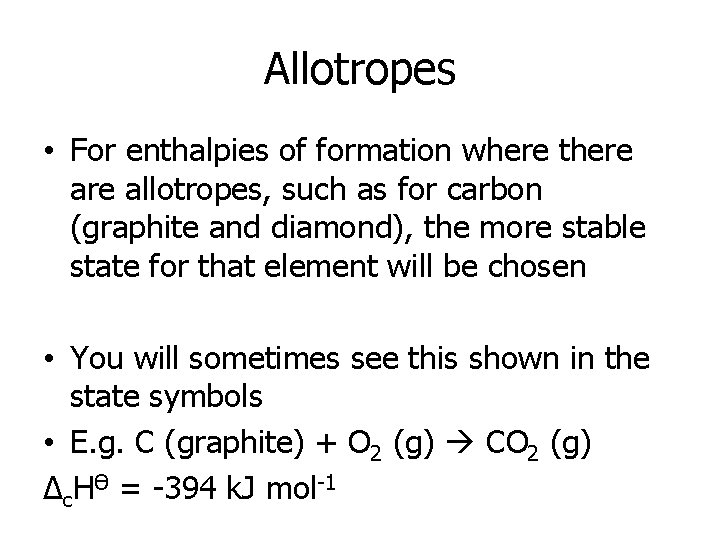
Allotropes • For enthalpies of formation where there allotropes, such as for carbon (graphite and diamond), the more stable state for that element will be chosen • You will sometimes see this shown in the state symbols • E. g. C (graphite) + O 2 (g) CO 2 (g) Δc. HӨ = -394 k. J mol-1

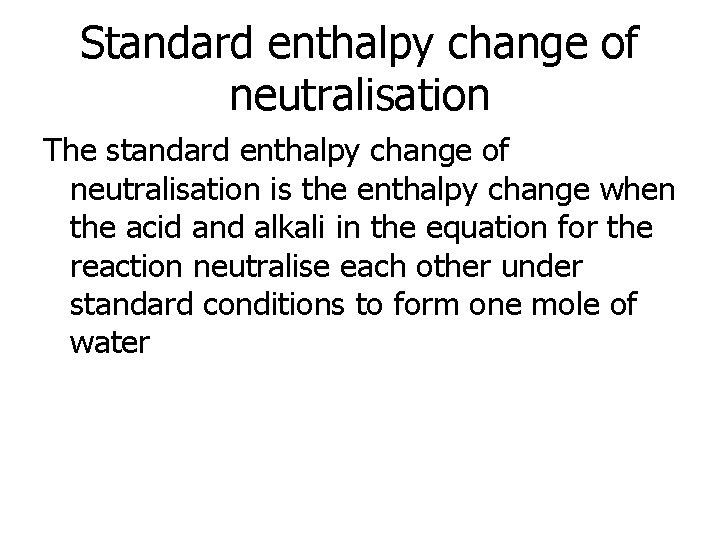
Standard enthalpy change of neutralisation The standard enthalpy change of neutralisation is the enthalpy change when the acid and alkali in the equation for the reaction neutralise each other under standard conditions to form one mole of water

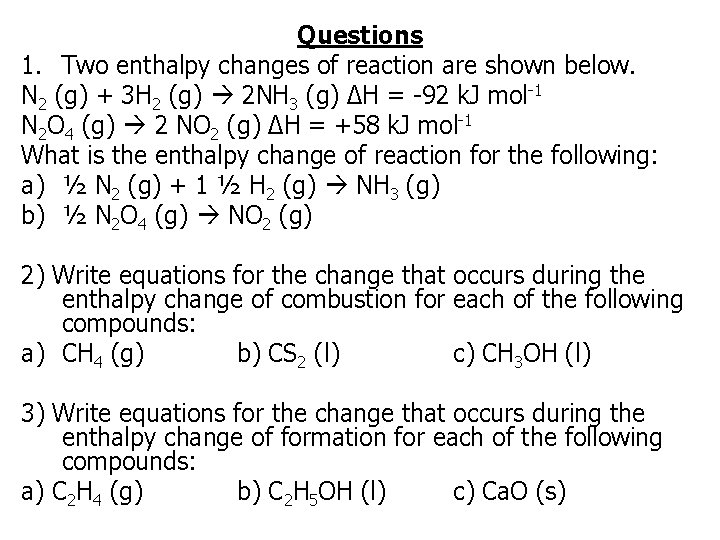
Questions 1. Two enthalpy changes of reaction are shown below. N 2 (g) + 3 H 2 (g) 2 NH 3 (g) ΔH = -92 k. J mol-1 N 2 O 4 (g) 2 NO 2 (g) ΔH = +58 k. J mol-1 What is the enthalpy change of reaction for the following: a) ½ N 2 (g) + 1 ½ H 2 (g) NH 3 (g) b) ½ N 2 O 4 (g) NO 2 (g) 2) Write equations for the change that occurs during the enthalpy change of combustion for each of the following compounds: a) CH 4 (g) b) CS 2 (l) c) CH 3 OH (l) 3) Write equations for the change that occurs during the enthalpy change of formation for each of the following compounds: a) C 2 H 4 (g) b) C 2 H 5 OH (l) c) Ca. O (s)

Determining Enthalpy Changes • Define and use the terms: – (i) standard conditions – (ii) enthalpy change of reaction – (iii) enthalpy change of formation – (iv) enthalpy change of combustion – (v) enthalpy change of neutralisation • Calculate enthalpy changes in k. J mol-1 from appropriate experimental results directly, including use of the relationship: energy change = mcΔT Both a sign and units are expected in the final answer

Measuring Energy Changes • • If we have an exothermic reaction, how would we measure how much energy it gave out to its surroundings? Firstly we must control what the surroundings are – Insulated from the outside world – A known amount – Can we measure energy directly?

Measuring Energy Changes • • If we know the temperature change of something, we would like to know how much energy it takes to cause that temperature change. We need to know the specific heat capacity of the substance.

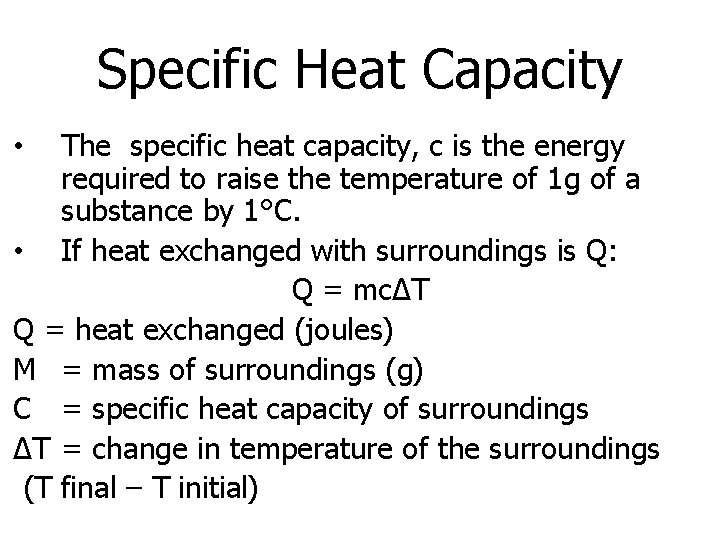
Specific Heat Capacity The specific heat capacity, c is the energy required to raise the temperature of 1 g of a substance by 1°C. • If heat exchanged with surroundings is Q: Q = mcΔT Q = heat exchanged (joules) M = mass of surroundings (g) C = specific heat capacity of surroundings ΔT = change in temperature of the surroundings (T final – T initial) •

Practice • Look at the example on the worksheet I have given you (This follows the same method as the enthalpy change of neutralisation example on page 247 of the text book) Note: your specification says to calculate the energy change in k. J mol-1 The units and sign are both expected in the final answer even if the change is +. You will lose marks for not including this

• Now look at the exam questions

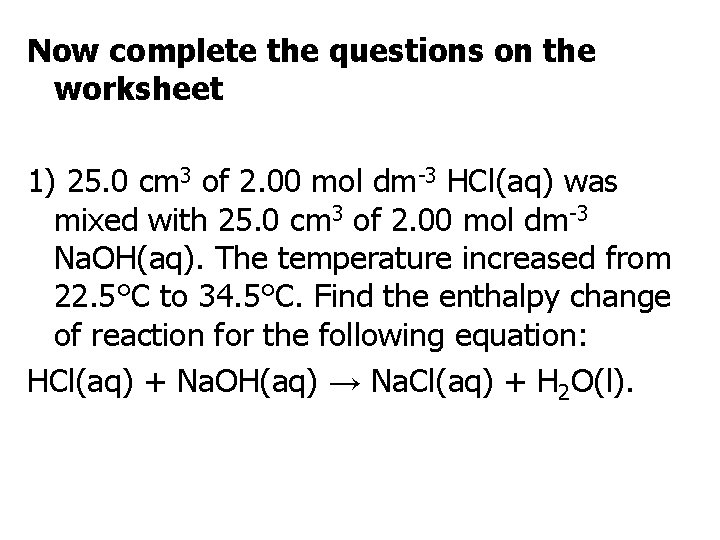
Now complete the questions on the worksheet 1) 25. 0 cm 3 of 2. 00 mol dm-3 HCl(aq) was mixed with 25. 0 cm 3 of 2. 00 mol dm-3 Na. OH(aq). The temperature increased from 22. 5°C to 34. 5°C. Find the enthalpy change of reaction for the following equation: HCl(aq) + Na. OH(aq) → Na. Cl(aq) + H 2 O(l).

0. 327 g of zinc powder was added to 55. 0 cm 3 of aqueous copper (II) sulfate at 22. 8 o. C. The temperature rose to 32. 3 o. C. The aqueous copper sulfate is in excess. Find the enthalpy change for the reaction for the following equation: Zn(s) + Cu. SO 4 (aq) Zn. SO 4 (aq) + Cu (s)

Determining Enthalpy Changes • Define and use the terms: – (i) standard conditions – (ii) enthalpy change of reaction – (iii) enthalpy change of formation – (iv) enthalpy change of combustion – (v) enthalpy change of neutralisation • Calculate enthalpy changes in k. J mol-1 from appropriate experimental results directly, including use of the relationship: energy change = mcΔT Both a sign and units are expected in the final answer

Determining Enthalpy Changes • Define and use the terms: – (i) standard conditions – (ii) enthalpy change of reaction – (iii) enthalpy change of formation – (iv) enthalpy change of combustion – (v) enthalpy change of neutralisation • Calculate enthalpy changes in k. J mol-1 from appropriate experimental results directly, including use of the relationship: energy change = mcΔT Both a sign and units are expected in the final answer