MATA KULIAH FARMAKOTERAPI I PHARMACEUTICAL CARE DRUG RELATED


































- Slides: 50

MATA KULIAH FARMAKOTERAPI I PHARMACEUTICAL CARE & DRUG RELATED PROBLEMS (DRP’s) Dhanang Prawira Nugraha Departemen Farmasi Klinis STIKes Karya Putra Bangsa Tulungagung dhanang. prawira. nugraha. apt@gmail. com © Dhanang | 2017 HAL |1

ARE YOU READY? ? © Dhanang | 2017 HAL|2

REFERENSI WAJIB © Dhanang | 2017 HAL|3

REFERENSI WAJIB 1. Dipiro, joseph T. , Talbert. Robert L. , Yee, Gary C. , Matzke, Gary R. , Wells, Barbara G. , Posey, L. Michael. , 2011, PHARMACOTHERAPY A PATHOPHISIOLOGIC APPROACH 8 TH EDITION, New York, Mc. Graw Hill 2. Alldredge, Brian L. , Corelli, Robin L. , Ernst Michael E. , Guglielmo B. Joseph. , Jacobson, Pamala A. , Kardjan, Wayne A. , Williams, Bradley R. , 2013, KODA-KIMBLE & YOUNG’S APPLIED THERAPEUTIC OF CLINICAL USE OF DRUGS. Philadelphia, Lippincot williams & wilkins, 3. Neal, Michael J. , 2012. , MEDICAL PHARMACOLOGY AT A GLANCE, london, wiley-blackwell 4. Brunton, Laurence L. , Chabner, Bruce A. , Knollman, Bjorn C. , 2012, GOODMAN GILLMAN THE PHARMACOLOGICAL BASIS OF THERAPEUTIC, 12 TH EDITION. , New York. , Mc. Graw Hill. 5. Cipolle, Robert J. Cipolle, Peter C. Morley, and Robert J. Cipolle. 2012. PHARMACEUTICAL CARE PRACTICE. New York: Mc. Graw-Hill. 6. Kasper, Dennis L. , Hauser, Stephen L. , Jameson, J. Larry. , Fauci, Anthony S. , Longo, Dan L. , Loscalzo, Joseph. , HARISSON’S PRINCIPLE INTERNAL MEDICINE 19 TH EDITION. , New York. , Mc. Graw Hill 7. Walker. , Roger. , Whittlesea, Cate. , 2012, CLINICAL PHARMACY AND THERAPEUTICS, London, Churchill livingstone. 8. Chisholm-Burns MA, Wells BG, Schwinghammer TL, Malone P, Kolesar J, Rotschafer J, et al. PHARMACOTHERAPY PRINCIPLES & PRACTICE. Mc. Graw-Hill New York; 2010. 9. Sukandar E yulinah, Andrajati R, Sigit JI, Adayana IK, Setiadi antonius A priyatno, Kusnandar. Iso Farmakoterapi. penerbit ISFI; 2008. 10. Widyawati, PRAKTEK FARMASI KLINIK FOKUS PADA PHARMACEUTICAL CARE, Brilian Internasional, 2014 11. Scwinghammer, Terry L. , Koehler, Julia M. , 2011, PHARMACOTHERAPY CASEBOOK A PATIENT-FOCUSED APPROACH 8 TH EDITION. , New York, Mc. Graw Hill 12. Dhillon , Soraya. , Raymond, Rebekah. , 2009, PHARMACY CASE STUDY, London, Pharmaceutical Press © Dhanang | 2017 HAL|4

OUTLINES • Sejarah & perkembangan Pharmaceutical Care • Pharmaceutical care • Assesment – DRUG RELATED Problem’s ASHP – DRUG RELATED Problem’s PCNE 6. 2 • Care plan – Monitoring – Therapy recomendation – Counseling • Follow up – Evaluation © Dhanang | 2017 HAL|5

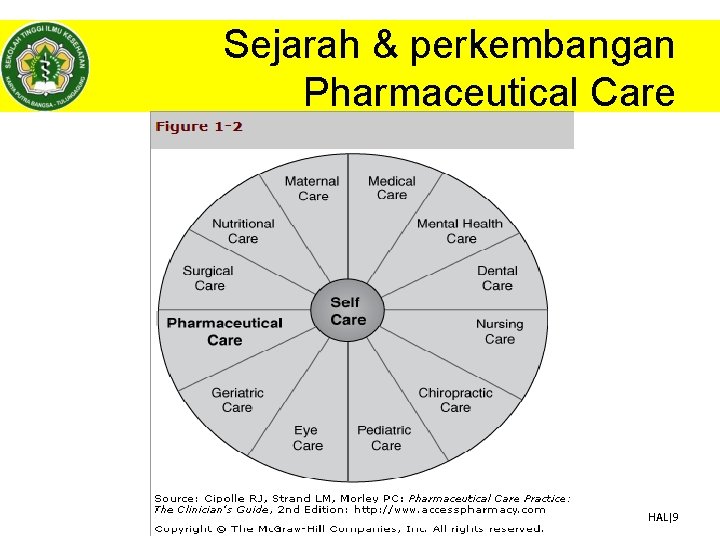
Sejarah & perkembangan Pharmaceutical Care • Pharmaceutical care lahir dari kebutuhan untuk mengkuantifikasi pelayanan kefarmasian, sehingga pelayanannya dapat terukur. • Menurut profesor linda strand pelayanan kefarmasian didefinisikan sebagai “responsible provision of drug therapy for the purpose of achieving definite outcomes than imporve a patient’s quality of life” • Definisi tersebut mengandung makna bahwa – Farmasis/apoteker memiliki tanggung jawab langsung terhadap pasien – Tujuan pengobatan harus dapat dinilai dan jelas – Outcomes (luaran) yang ingin dicapai bukan hanya kesembuhan semata akan tetapi lebih kepada bagaimana meningkatkan kualitas hidup pasien © Dhanang | 2017 HAL|6

Sejarah & perkembangan Pharmaceutical Care • Menurut PCNE tahun 2013 “pharmacist’s contribution to the care of individuals in order to optimise medicine use and improve health outcomes” • Hal ini mengandung makna bahwa seorang farmasis/apoteker – Merupakan bagian dari tim kesehatan yang berkontribusi dalam perawatan pasien – Mengopitmalkan terapi pasien dan meminimalkan resiko atau efek samping – Outcomes (luaran) terapi pasien dengan adanya terapi yang optimal diharapkan akan meningkatkan kualitas hidup dari pasien © Dhanang | 2017 HAL|7

Sejarah & perkembangan Pharmaceutical Care • Pelayanan kesehatan semakin kompleks dan terjadinya evolusi terhadap pelayanan kefarmasian sehingga tidak jarang munculnya kesalahpahaman antara profesi kesehatan lainnya – pharmaceutical care tidak menggantikan dokter dan profesi lainnya – Untuk memenuhi kebutuhan dalam sistem pelayanan kesehatan lainya • • • Banyaknya obat untuk seorang pasien Ledakan informasi obat Banyaknya obat yang beredar Meningkatnya kompleksitas terapi Adanya drug related problems Tingginya biaya pengobatan dan resiko akibat penggunaan obat yang tidak tepat © Dhanang | 2017 HAL|8

Sejarah & perkembangan Pharmaceutical Care © Dhanang | 2017 HAL|9

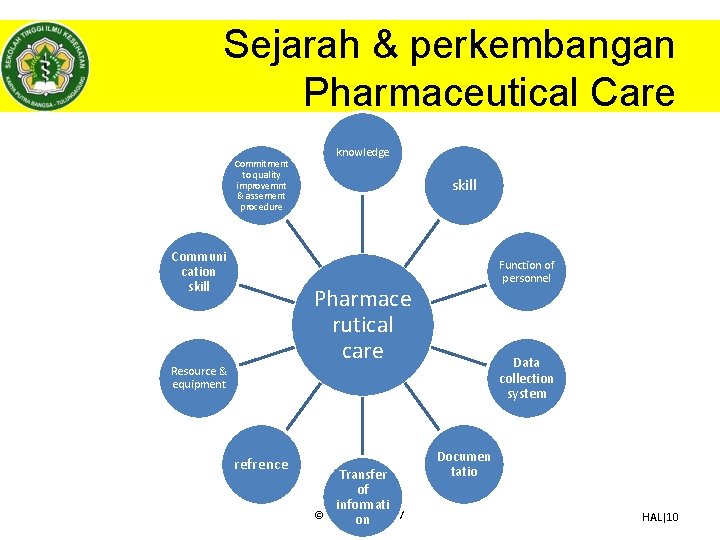
Sejarah & perkembangan Pharmaceutical Care Commitment to quality improvemnt & assement procedure Communi cation skill knowledge skill Function of personnel Pharmace rutical care Resource & equipment refrence Transfer of informati © Dhanang on | 2017 Data collection system Documen tatio HAL|10

Pharmaceutical Care Skema Pharmaceutical Care • Assesment Care plan Follow up Drug Related Problems monitoring Evaluation database pasien • Subjektif • Objektif • Problem medik • Terapi Therapy recomendation © Counseling Dhanang | 2017 HAL|11

ASSESSMENT - Definisi Drug Related Problem’s & © Dhanang | 2017 HAL|12

ASSESSMENT - Definisi Drug Related Problem’s • Menurut PCNE (Pharmaceutical Care Network Europe) drug related problem’s didefinisikan sebagai “is an event or circumstance involving drug therapy that actually or potentially interferes with desired health outcomes. ” • Menurut ASHP (American Society of Health-System Pharmacists) “A drug therapy problem is any undesirable event experienced by a patient which involves, or is suspected to involve, drug therapy, and that interferes with achieving the desired goals of therapy. ” – Drug Related Problem’s menurut ASHP di sebut juga sebagai Drug Therapy Problems’s © Dhanang | 2017 HAL|13

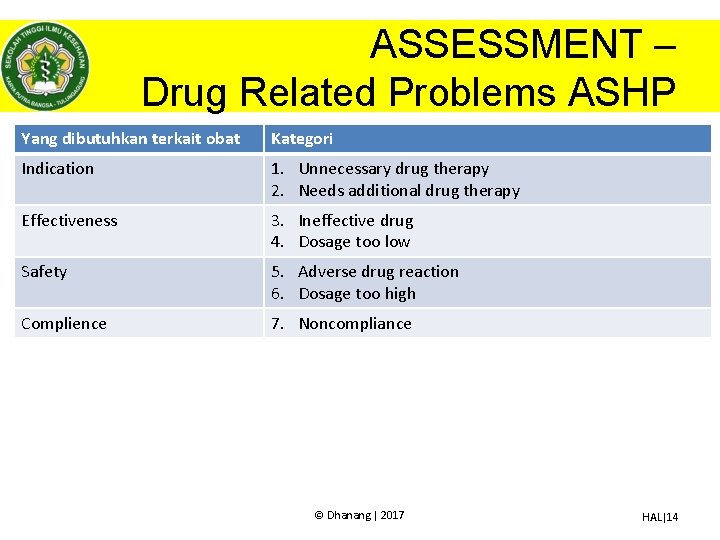
ASSESSMENT – Drug Related Problems ASHP Yang dibutuhkan terkait obat Kategori Indication 1. Unnecessary drug therapy 2. Needs additional drug therapy Effectiveness 3. Ineffective drug 4. Dosage too low Safety 5. Adverse drug reaction 6. Dosage too high Complience 7. Noncompliance © Dhanang | 2017 HAL|14

ASSESSMENT – Drug Related Problems ASHP 1. Unnecessary drug therapy • There is no valid medical indication for the drug therapy at this time. • Multiple drug products are being used for a condition that requires single drug therapy. • The medical condition is more appropriately treated with nondrug therapy • Drug therapy is being taken to treat an avoidable adverse reaction associated with another medication. • Drug abuse, alcohol use, or smoking is causing the problem. © Dhanang | 2017 HAL|15

ASSESSMENT – Drug Related Problems ASHP 2. Needs additional drug therapy • A medical condition requires the initiation of drug therapy. • Preventive drug therapy is required to reduce the risk of developing a new condition. • A medical condition requires additional pharmacotherapy to attain synergistic or additive effects. © Dhanang | 2017 HAL|16

ASSESSMENT – Drug Related Problems ASHP 3. Ineffective drug • The drug is not the most effective for the medical problem. • The medical condition is refractory to the drug product. • The dosage form of the drug product is inappropriate. • The drug product is not an effective product for the indication being treated. © Dhanang | 2017 HAL|17

ASSESSMENT – Drug Related Problems ASHP 4. Dosage too low • The dose is too low to produce the desired response. • The dosage interval is too infrequent to produce the desired response. • A drug interaction reduces the amount of active drug available. • The duration of drug therapy is too short to produce the desired response. © Dhanang | 2017 HAL|18

ASSESSMENT – Drug Related Problems ASHP 5. Adverse drug reaction • • • The drug product causes an undesirable reaction that is not dose-related. A safer drug product is required due to risk factors. A drug interaction causes an undesirable reaction that is not dose-related. The dosage regimen was administered or changed too rapidly. The drug product causes an allergic reaction. The drug product is contraindicated due to risk factors. © Dhanang | 2017 HAL|19

ASSESSMENT – Drug Related Problems ASHP 6. Dosage too high Dose is too high. The dosing frequency is too short. The duration of drug therapy is too long. A drug interaction occurs resulting in a toxic reaction to the drug product. • The dose of the drug was administered too rapidly. • • © Dhanang | 2017 HAL|20

ASSESSMENT – Drug Related Problems ASHP 7. Noncompliance The patient does not understand the instructions. The patient prefers not to take the medication. The patient forgets to take the medication. The drug product is too expensive for the patient. The patient cannot swallow or self-administer the drug product appropriately. • The drug product is not available for the patient. • • • © Dhanang | 2017 HAL|21

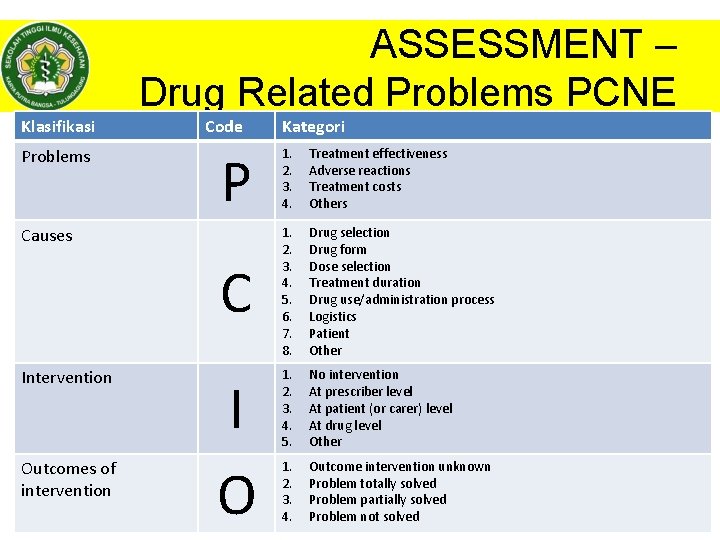
ASSESSMENT – Drug Related Problems PCNE Klasifikasi Problems Code P 1. 2. 3. 4. Treatment effectiveness Adverse reactions Treatment costs Others C 1. 2. 3. 4. 5. 6. 7. 8. Drug selection Drug form Dose selection Treatment duration Drug use/administration process Logistics Patient Other 1. 2. 3. 4. 5. No intervention At prescriber level At patient (or carer) level At drug level Other 1. 2. 3. 4. Outcome intervention unknown Problem totally solved Problem partially solved © Dhanang Problem not| 2017 solved Causes Intervention Outcomes of intervention Kategori I O HAL|22

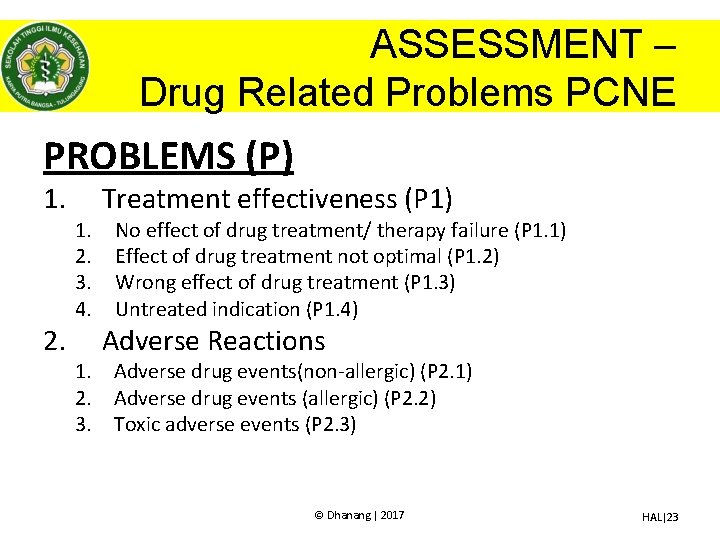
ASSESSMENT – Drug Related Problems PCNE PROBLEMS (P) 1. 2. 1. 2. 3. 4. Treatment effectiveness (P 1) No effect of drug treatment/ therapy failure (P 1. 1) Effect of drug treatment not optimal (P 1. 2) Wrong effect of drug treatment (P 1. 3) Untreated indication (P 1. 4) Adverse Reactions 1. Adverse drug events(non-allergic) (P 2. 1) 2. Adverse drug events (allergic) (P 2. 2) 3. Toxic adverse events (P 2. 3) © Dhanang | 2017 HAL|23

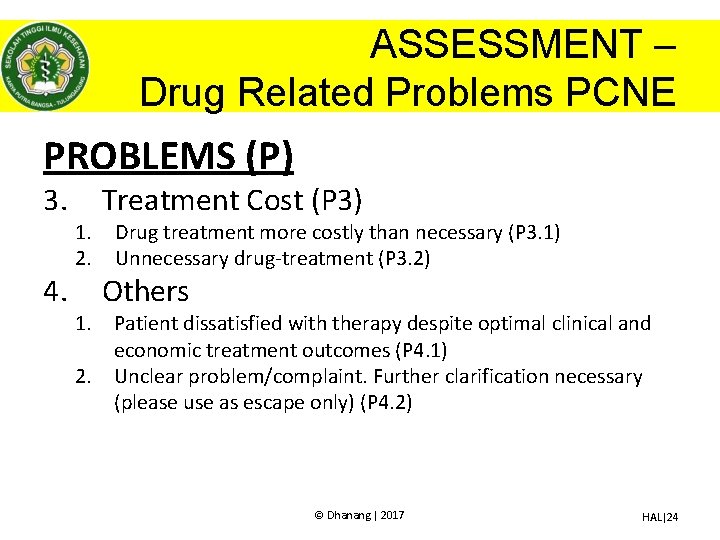
ASSESSMENT – Drug Related Problems PCNE PROBLEMS (P) 3. 4. 1. 2. Treatment Cost (P 3) Drug treatment more costly than necessary (P 3. 1) Unnecessary drug-treatment (P 3. 2) Others 1. Patient dissatisfied with therapy despite optimal clinical and economic treatment outcomes (P 4. 1) 2. Unclear problem/complaint. Further clarification necessary (please use as escape only) (P 4. 2) © Dhanang | 2017 HAL|24

ASSESSMENT – Drug Related Problems PCNE CAUSES (C) 1. 2. 1. 2. 3. 4. 5. 6. 7. 8. 9. 1. Drug selection (C 1) Inappropriate drug (incl. contra-indicated) (C 1. 1) No indication for drug (C 1. 2) Inappropriate combination of drugs, or drugs and food (C 1. 3) Inappropriate duplication of therapeutic group or active ingredient (C 1. 4) Indication for drug-treatment noticed (C 1. 5) Too many drugs prescribed for indication (C 1. 6) More cost-effective drug available (C 1. 7) Synergistic/preventive drug required and not given (C 1. 8) New indication for drug treatment presented (C 1. 9) Drug Form Inappropiate drug form (C 2. 1) © Dhanang | 2017 HAL|25

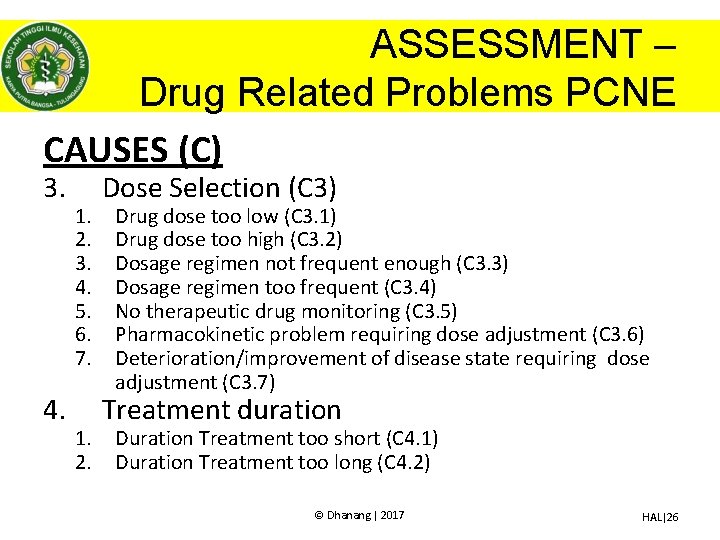
ASSESSMENT – Drug Related Problems PCNE CAUSES (C) 3. 4. 1. 2. 3. 4. 5. 6. 7. 1. 2. Dose Selection (C 3) Drug dose too low (C 3. 1) Drug dose too high (C 3. 2) Dosage regimen not frequent enough (C 3. 3) Dosage regimen too frequent (C 3. 4) No therapeutic drug monitoring (C 3. 5) Pharmacokinetic problem requiring dose adjustment (C 3. 6) Deterioration/improvement of disease state requiring dose adjustment (C 3. 7) Treatment duration Duration Treatment too short (C 4. 1) Duration Treatment too long (C 4. 2) © Dhanang | 2017 HAL|26

ASSESSMENT – Drug Related Problems PCNE CAUSES (C) 5. 6. 1. 2. 3. 4. 5. 6. 7. Drug use process (C 5) Inappropriate timing of administration and/or dosing intervals (C 5. 1) Drug underused/ under-administered (deliberately) (C 5. 1) Drug overused/ over-administered (deliberately) (C 5. 2) Drug not taken/administered at all (C 5. 3) Wrong drug taken/administered (C 5. 4) Drug abused (unregulated overuse) (C 5. 5) Patient unable to use drug/form as directed (C 5. 6) Logistic (C 6. 1) 1. Prescribed drug not available (C 6. 1) 2. Prescribing error (necessary information missing) (C 6. 2) 3. Dispensing error (wrong drug or dose dispensed) (C 6. 3) © Dhanang | 2017 HAL|27

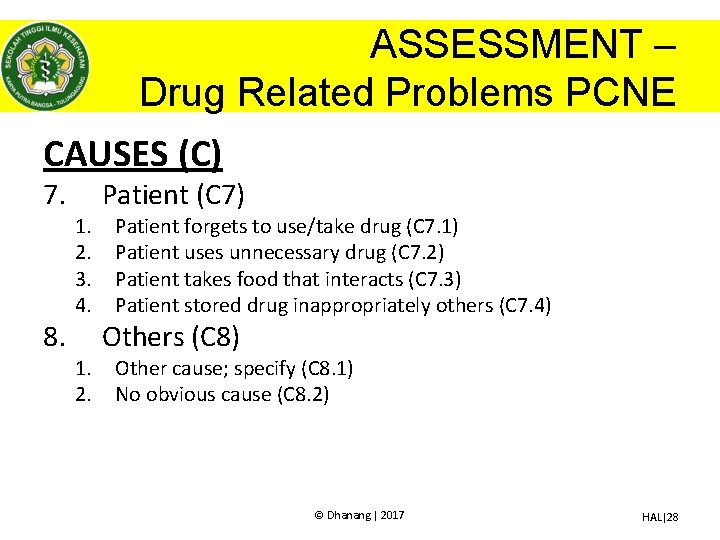
ASSESSMENT – Drug Related Problems PCNE CAUSES (C) 7. 8. 1. 2. 3. 4. 1. 2. Patient (C 7) Patient forgets to use/take drug (C 7. 1) Patient uses unnecessary drug (C 7. 2) Patient takes food that interacts (C 7. 3) Patient stored drug inappropriately others (C 7. 4) Others (C 8) Other cause; specify (C 8. 1) No obvious cause (C 8. 2) © Dhanang | 2017 HAL|28

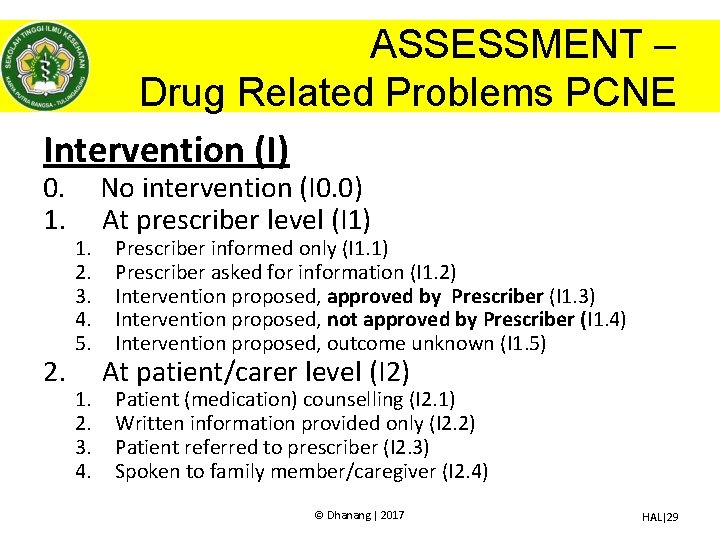
ASSESSMENT – Drug Related Problems PCNE Intervention (I) 0. 1. 2. 1. 2. 3. 4. 5. 1. 2. 3. 4. No intervention (I 0. 0) At prescriber level (I 1) Prescriber informed only (I 1. 1) Prescriber asked for information (I 1. 2) Intervention proposed, approved by Prescriber (I 1. 3) Intervention proposed, not approved by Prescriber (I 1. 4) Intervention proposed, outcome unknown (I 1. 5) At patient/carer level (I 2) Patient (medication) counselling (I 2. 1) Written information provided only (I 2. 2) Patient referred to prescriber (I 2. 3) Spoken to family member/caregiver (I 2. 4) © Dhanang | 2017 HAL|29

ASSESSMENT – Drug Related Problems PCNE Intervention (I) 3. 4. 1. 2. 3. 4. 5. 6. 1. 2. At drug level (I 3) Dose change to. . . . (I 3. 1) Dosage changed to. . . . (I 3. 2) Formulation changed to. . . . (I 3. 3) Instructions for use changed to. . . . (I 3. 4) Drug stopped (I 3. 5) New drug started (I 3. 6) Other intervention or activity (I 2) Other intervention (specify) (I 4. 1) Side effect reported to authorities (4. 2) © Dhanang | 2017 HAL|30

ASSESSMENT – Drug Related Problems PCNE Outcomes (O) 0. 1. 2. 3. Not Known (O 0. 0) Solved (O 1) Partially solved (O 2) Not solved (O 3) 1. Problem not solved, lack of cooperation of patient (O 4. 1) 2. Problem not solved, lack of cooperation of prescriber (O 4. 2) 3. Problem not solved, intervention not effective (O 4. 3) 4. No need or possibility to solve problem (O 4. 4) © Dhanang | 2017 HAL|31

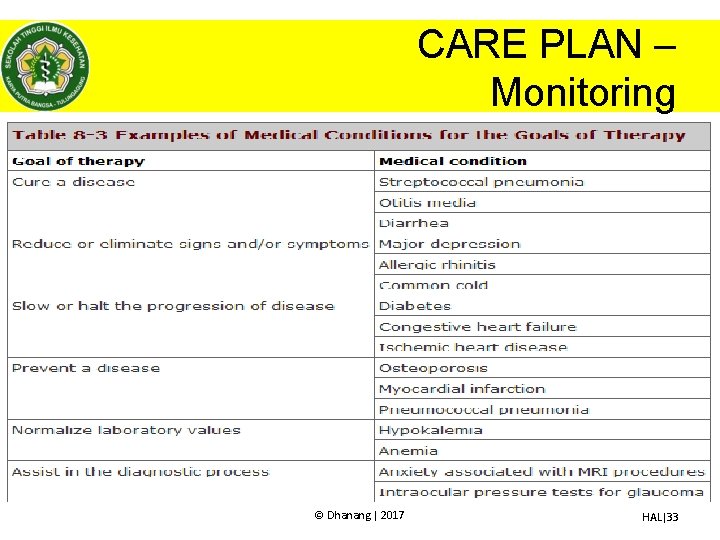
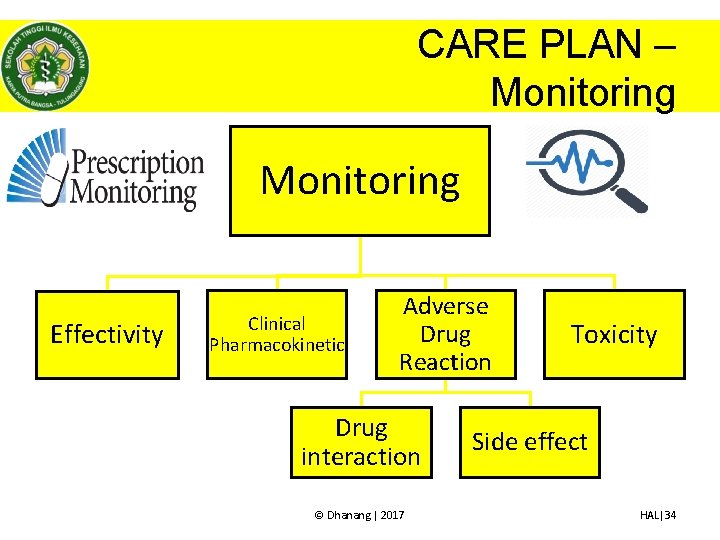
CARE PLAN – Monitoring • Monitoring terapi menjadi tanggung jawab farmasis/apoteker karena terkait dengan – Pencapaian outcome – Identifikasi DRP • Monitoring yang dilakukan oleh farmasis/apoteker menantau terapi berdasarkan terapi obat yang diberikan memantau (mengamati, mencatat) data dan kondisi klinis terkait obat saja • Monitoring dilakukan sesuai dengan tujuan dari terapi pasien © Dhanang | 2017 HAL|32

CARE PLAN – Monitoring © Dhanang | 2017 HAL|33

CARE PLAN – Monitoring Effectivity Clinical Pharmacokinetic Adverse Drug Reaction Drug interaction © Dhanang | 2017 Toxicity Side effect HAL|34

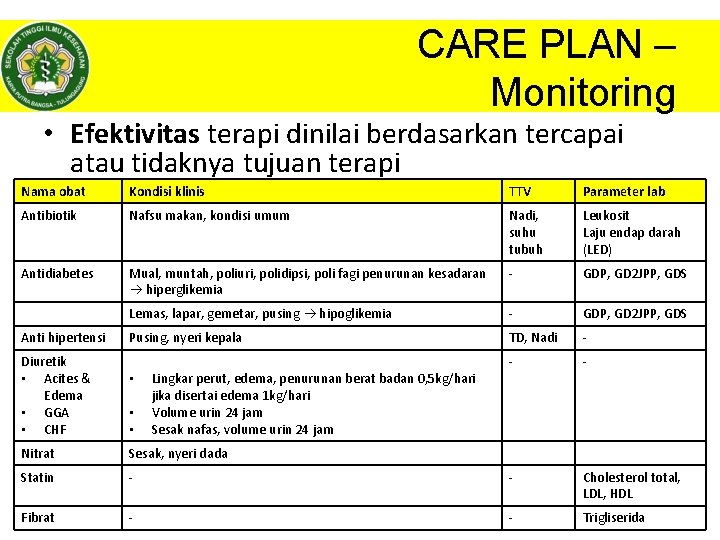
CARE PLAN – Monitoring • Efektivitas terapi dinilai berdasarkan tercapai atau tidaknya tujuan terapi Nama obat Kondisi klinis TTV Parameter lab Antibiotik Nafsu makan, kondisi umum Nadi, suhu tubuh Leukosit Laju endap darah (LED) Antidiabetes Mual, muntah, poliuri, polidipsi, poli fagi penurunan kesadaran hiperglikemia - GDP, GD 2 JPP, GDS Lemas, lapar, gemetar, pusing hipoglikemia - GDP, GD 2 JPP, GDS Pusing, nyeri kepala TD, Nadi - - Cholesterol total, LDL, HDL - Trigliserida. HAL|35 Anti hipertensi Diuretik • Acites & Edema • GGA • CHF • • • Lingkar perut, edema, penurunan berat badan 0, 5 kg/hari jika disertai edema 1 kg/hari Volume urin 24 jam Sesak nafas, volume urin 24 jam Nitrat Sesak, nyeri dada Statin - Fibrat - © Dhanang | 2017

CARE PLAN – Monitoring • Clinical pharmacokinetic atau Monitoring farmakokinetik (Therapeutic drug monitoring) pemantauan terkait kadar obat dalam plasma terkait penilaian efektivitas terapi maupun efek samping obat. • Disarankan untuk obat dengan indeks terapi sempit seperti – – – – Digoxin Phenitoin Carbamazepin Asam valproat Aminoglikosida Amfoterisin B Dll • TDM direkomendasikan pada keadaan – Menunjukkan respon yang tidak lazim – Adanya ESO/toksisitas – Respon yang minimal pada dosis besar © Dhanang | 2017 HAL|36

CARE PLAN – Monitoring • Monitoring Adverse drug reaction – monitoring efek samping obat • Tugas apoteker/farmasis untuk dapat mengidentifikasi ESO yang potensial yang akan terjadi sehingga dapat dicegah dan menindaklanjuti ESO yang aktual yang terjadi, sehingga penting bagi apoteker/farmasis untuk mengetahui tanda dan gejala ESO. • ESO yang perlu diperhatikan oleh apoteker/farmasis v. ESO yang berakibat fatal v. ESO dengan frekuensi yang tinggi © Dhanang | 2017 HAL|37

CARE PLAN – Monitoring Parameter monitoring ESO beberapa golongan obat Nama obat Kondisi klinis TTV Parameter lab Penisilin, sefalosporin dan derivatnya Tanda alergi (rash, urtikaria pruritus) Suhu tubuh Eosinofil Anttidiabetes, insulin Tanda hipoglikemia - GDP, GD 2 JPP, GDS Captopril Batuk kering, rash, pruritus - Kreatinin, BUN, bilirubin, kalium Beta-blocker Persisten bradikardi, dizziness, cold extrimites Nadi - Diuretik pada GGA Hiperuricemia, blurred vision, Tinnitus Tekanan darah Asam urat, gula Statin Myostitis, rhabdomyolisis - SGPT & SGOT Fibrat Myostitis, rhabdomyolisis - SGPT & SGOT Lansoprazole Bone fracture - - Salbutamol Tremor, mual RR - © Dhanang | 2017 HAL|38

CARE PLAN – Monitoring • Monitoring Adverse drug reaction – Monitoring interaksi obat • Interaksi dapat bersifat potensial maupun aktual. • Interaksi dikenali setelah muncul gejala dan tanda yang menetap pada pasien • Interaksi obat dapat mempengaruhi pemeriksaan laboratorium • Interaksi yang perlu diwaspadai adalah interaksi yang “clinically significant drug interaction” © Dhanang | 2017 HAL|39

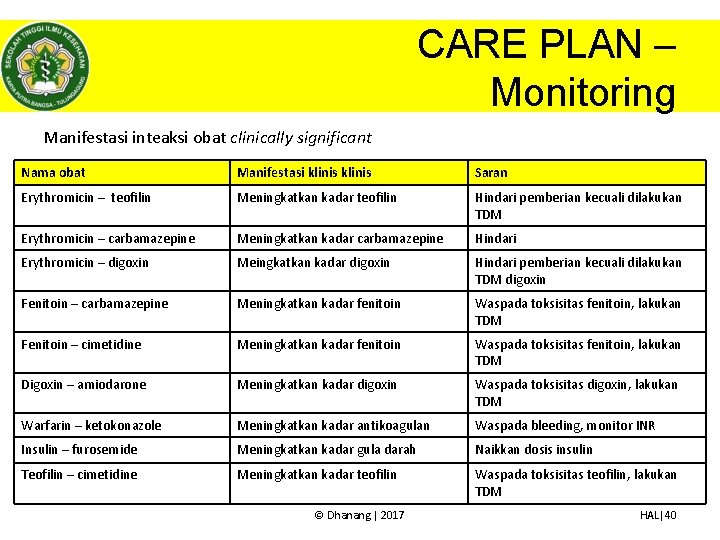
CARE PLAN – Monitoring Manifestasi inteaksi obat clinically significant Nama obat Manifestasi klinis Saran Erythromicin – teofilin Meningkatkan kadar teofilin Hindari pemberian kecuali dilakukan TDM Erythromicin – carbamazepine Meningkatkan kadar carbamazepine Hindari Erythromicin – digoxin Meingkatkan kadar digoxin Hindari pemberian kecuali dilakukan TDM digoxin Fenitoin – carbamazepine Meningkatkan kadar fenitoin Waspada toksisitas fenitoin, lakukan TDM Fenitoin – cimetidine Meningkatkan kadar fenitoin Waspada toksisitas fenitoin, lakukan TDM Digoxin – amiodarone Meningkatkan kadar digoxin Waspada toksisitas digoxin, lakukan TDM Warfarin – ketokonazole Meningkatkan kadar antikoagulan Waspada bleeding, monitor INR Insulin – furosemide Meningkatkan kadar gula darah Naikkan dosis insulin Teofilin – cimetidine Meningkatkan kadar teofilin Waspada toksisitas teofilin, lakukan TDM © Dhanang | 2017 HAL|40

CARE PLAN – Monitoring • Monitoring toksisitas terjadi karena dosis obat yang berlebihan atau terjadinya interaksi. Untuk mengetahui toksistas perlu diketahui tanda-tanda klinis maupun laboratorium Nama obat Tanda toksisitas Fenitoin Ataksia, nystagmus, blurred vision, slurred speech, dizzines, drowsiness, confusion, mood, changes, kadar plasma fenitoin > 100 µg kematian Teofilin/aminofilin Muntah, agitasi, konvulsi, takikardi, pupil, dilatasi, tremor, insomnia, iritabilitas, hipokalemia Digoxin Perubahan ECG hiperkalemia, mual, muntah, blurred vision, perubahan tingkah laku, confusion, hearth block, Warfarin Perdarahan internal, coma, angina, nyeri abdomen, nyeri otot Paracetamol Tender hepatic edge, jaundice, coagulopathy, hypoglicemia, hepatic enlophaty Benzodizepine Dizzines, confusion, drowziines, unresponziveness, anxiety, , agitation © Dhanang | 2017 HAL|41

CARE PLAN – Recommendations Therapy • The practitioner develops a plan of care that includes interventions to: – Resolve drug therapy problems – Achieve goals of therapy – Prevent drug therapy problems. • Measurement Criteria 1. Each intervention is individualized to the patient's conditions, needs, and drug therapy problems. 2. All appropriate therapeutic alternatives to resolve drug therapy problems are considered, and the best are selected. 3. The plan is developed in collaboration with the patient, his/her family and/or care-givers, and health care providers, when appropriate. 4. All interventions are documented. 5. The plan provides for continuity of care by including a schedule for continuous follow-up evaluation. © Dhanang | 2017 HAL|42

EVALUATION – Counseling • Konseling adalah suatu kegiatan bertemu dan berdiskusinya seseorang yang membutuhkan (klien) dan seseorang yang memberikan (konselor) dukungan dorongan sedemikian rupa sehingga klien memperoleh keyakinan akan kemampuannya dalam pemecahan masalah. • Konseling yang diberikan atas inisiatif langsung dari Apoteker disebut konseling aktif • konseling terjadi jika pasien datang untuk berkonsultasi pada apoteker untuk mendapatkan penjelasan tentang segala sesuatu yang berhubungan dengan obat dan pengobatan, bentuk konseling seperti ini disebut konseling pasif. • Enam komponen konseling minimal yaitu: – – – Nama obat, jumlahnya dan indikasinya Aturan pakai, cara dan lama pemakaian Interaksi obat Efek samping obat Pengaruh terhadap pola hidup, pola makan Cara penyimpanan © Dhanang | 2017 HAL|43

CARE PLAN – Evaluasi • The Practitioner Develops a Schedule to Follow-Up and Evaluate the Effectiveness of Drug Therapies and Assess Any Adverse Events Experienced by the Patient. • Measurement Criteria 1. 2. 3. 4. The clinical and laboratory parameters to evaluate effectiveness are established, and a timeframe for collecting the relevant information is selected. The clinical and laboratory parameters that reflect the safety of the patient's medications are selected, and a timeframe for collecting the relevant information is determined. A schedule for the follow-up evaluation is established with the patient. The plan for follow-up evaluation is documented. © Dhanang | 2017 HAL|44

Contoh Kasus • Tn A, Usia: 61 th, BB: 60 kg TB: 165 cm. datang ke klinik dengan keluhan panas dan nyeri pada ulu hati dan mual disertai muntah, pasien muntah dengan warna coklat kehitam-hitaman. Pasien rutin mengkonsumsi “jamu” yang dibeli dipasar untuk mengobati pegallinunya karena pasien bekerja sebagai tukang batu. • Dokter mendiagnosa pasien dengan peptic ulcer disease induced NSAID • TTV: TD 130/90 mm. Hg, RR: 20 x per menit, HR nadi: 80 X/menit, suhu tubuh 37, 50 C • Dokter meresepkan: Novalgin 500 mg 3 x 1, antasida 3 x 3 tab, ranitidine 1 x 1, lansoprazole caps 3 x 1, ondansentrone tab 4 mg 3 x 1, amoxsan tab 500 mg 4 x 1 • Saat mengambil obat apoteker menanyakan alergi obat kepada pasien dan pasien mengatakan punya alergi terhadap penisilin © Dhanang | 2017 HAL|45

Contoh Kasus • Assesment – Database pasien • Tn A, Usia: 61 th, BB: 60 kg TB: 165 cm – Subjektif • keluhan panas dan nyeri pada ulu hati dan mual disertai muntah, pasien muntah dengan warna coklat kehitam-hitaman • pasien muntah dengan warna coklat kehitam-hitaman. Pasien rutin mengkonsumsi “jamu” yang dibeli dipasar • Pasien memiliki alergi penisilin – Objektif • Tidak ada – Problem medik • Peptic ulcer disease – Terapi • • • methampyrone 500 mg 3 x 1, antasida 3 x 3 tab ranitidine 3 x 1 lansoprazole 3 x 1 ondansentrone tablet 4 mg 3 x 1 amoxicilin 500 mg 4 x 1 © Dhanang | 2017 HAL|46

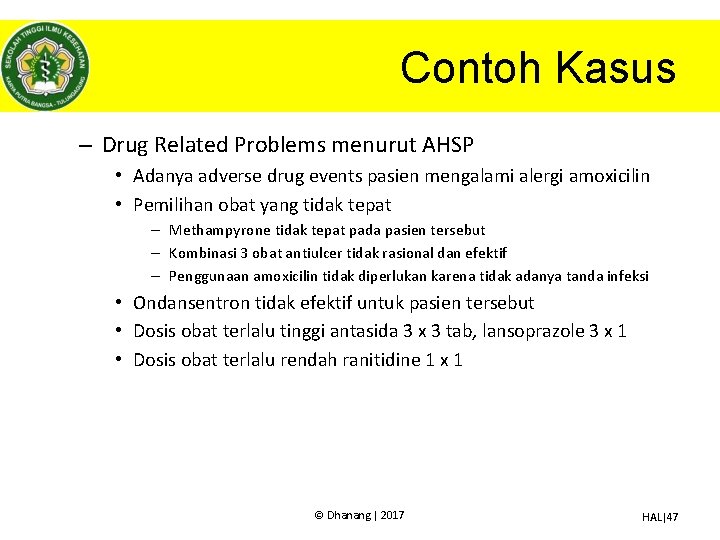
Contoh Kasus – Drug Related Problems menurut AHSP • Adanya adverse drug events pasien mengalami alergi amoxicilin • Pemilihan obat yang tidak tepat – Methampyrone tidak tepat pada pasien tersebut – Kombinasi 3 obat antiulcer tidak rasional dan efektif – Penggunaan amoxicilin tidak diperlukan karena tidak adanya tanda infeksi • Ondansentron tidak efektif untuk pasien tersebut • Dosis obat terlalu tinggi antasida 3 x 3 tab, lansoprazole 3 x 1 • Dosis obat terlalu rendah ranitidine 1 x 1 © Dhanang | 2017 HAL|47

Contoh Kasus • Care plan – Monitoring • efektivitas: mual muntah, nyeri di ulu hati • Efek samping obat: – Rekomendasi terapi • Sebaiknya antibiotik dihentikan karena tidak adanya tanda infeksi dan pasien mengalami alergi penisilin • Untuk nyerinya pasien dapat digantikan dengan ibuprofen yang relatif lebih aman bagi lambung • Penggunaan lansoprazole lebih efektif dalam pengobatan peptic ulcer yang disertai dengan perdararahan dibanding dengan ranitidine • Penggunaan ondansetron sebaiknya dihentikan karena tidak efektif bagi pasien • Lansoprazole dapat diberikan 2 kali sehari selama 1 bulan untuk mengobati peptic ulcer terkait dengan NSAID – Konseling • • • Nama obat, jumlahnya dan indikasinya Aturan pakai, cara dan lama pemakaian Interaksi obat Efek samping obat Pengaruh terhadap pola hidup, pola makan Cara penyimpanan © Dhanang | 2017 HAL|48

Contoh Kasus • Follow up – Evaluasi efektivitas terapi – Evaluasi ADR © Dhanang | 2017 HAL|49

© Dhanang | 2017 HAL|50
 Farmakoterapi diare
Farmakoterapi diare Farmakoterapi rasional dan irasional
Farmakoterapi rasional dan irasional Deskripsi mata kuliah etika profesi
Deskripsi mata kuliah etika profesi Materi pengembangan diri mahasiswa
Materi pengembangan diri mahasiswa Notanerd
Notanerd Mata kuliah ekonomi pembangunan ub
Mata kuliah ekonomi pembangunan ub Mata kuliah pelayanan prima
Mata kuliah pelayanan prima Contoh metode keterikatan dana
Contoh metode keterikatan dana Kurikulum teknik informatika gunadarma
Kurikulum teknik informatika gunadarma Mata kuliah metode penelitian teknik informatika
Mata kuliah metode penelitian teknik informatika Mata kuliah ilmu kelautan unpad
Mata kuliah ilmu kelautan unpad Deskripsi mata kuliah ekonomi mikro
Deskripsi mata kuliah ekonomi mikro Tujuan creative writing
Tujuan creative writing Cpmk
Cpmk Cjr mata kuliah kewirausahaan
Cjr mata kuliah kewirausahaan Mata kuliah testing dan implementasi sistem
Mata kuliah testing dan implementasi sistem Deskripsi mata kuliah statistik
Deskripsi mata kuliah statistik Silabus mata kuliah sejarah pendidikan islam
Silabus mata kuliah sejarah pendidikan islam Mata kuliah keamanan sistem informasi
Mata kuliah keamanan sistem informasi Contoh rekonstruksi mata kuliah
Contoh rekonstruksi mata kuliah Mata kuliah ekonomi islam ub
Mata kuliah ekonomi islam ub Tujuan pemrograman visual
Tujuan pemrograman visual 3 pilar kunci guru profesional
3 pilar kunci guru profesional Mata kuliah keamanan jaringan
Mata kuliah keamanan jaringan Pengertian seminar akuntansi
Pengertian seminar akuntansi Mata kuliah penyuntingan
Mata kuliah penyuntingan Kurikulum institusional adalah
Kurikulum institusional adalah Materi kuliah ilmu alamiah dasar semester 2
Materi kuliah ilmu alamiah dasar semester 2 Materi kuliah fisika lingkungan
Materi kuliah fisika lingkungan Pengantar aplikasi komputer (spss)
Pengantar aplikasi komputer (spss) Mata kuliah sistem produksi
Mata kuliah sistem produksi Materi kuliah sistem informasi manajemen
Materi kuliah sistem informasi manajemen Deskripsi mata kuliah pengantar bisnis
Deskripsi mata kuliah pengantar bisnis Relevansi mata kuliah menyimak dengan berbicara
Relevansi mata kuliah menyimak dengan berbicara Materi kuliah manajemen keuangan
Materi kuliah manajemen keuangan Baja prategang
Baja prategang Mata kuliah struktur beton
Mata kuliah struktur beton Uas perilaku organisasi
Uas perilaku organisasi Mata kuliah manajemen proyek sistem informasi
Mata kuliah manajemen proyek sistem informasi Pertanyaan tentang tugas-tugas perkembangan peserta didik
Pertanyaan tentang tugas-tugas perkembangan peserta didik Bahan i
Bahan i Notasi erd
Notasi erd Mata kuliah mercu buana
Mata kuliah mercu buana Mata kuliah pengantar arsitektur
Mata kuliah pengantar arsitektur Ocular inserts
Ocular inserts Mata kuliah sik
Mata kuliah sik Silabus mata kuliah seminar proposal skripsi
Silabus mata kuliah seminar proposal skripsi Mata kuliah pengantar arsitektur
Mata kuliah pengantar arsitektur Perencanaan dan pengendalian produksi
Perencanaan dan pengendalian produksi Mata kuliah administrasi perpajakan ui
Mata kuliah administrasi perpajakan ui Mata kuliah pip
Mata kuliah pip