Massachusetts Society of Pathology 2017 Molecular Pathology A






- Slides: 65

Massachusetts Society of Pathology 2017: Molecular Pathology A. John Iafrate MD-Ph. D Department of Pathology Massachusetts General Hospital Boston, MA aiafrate@partners. org

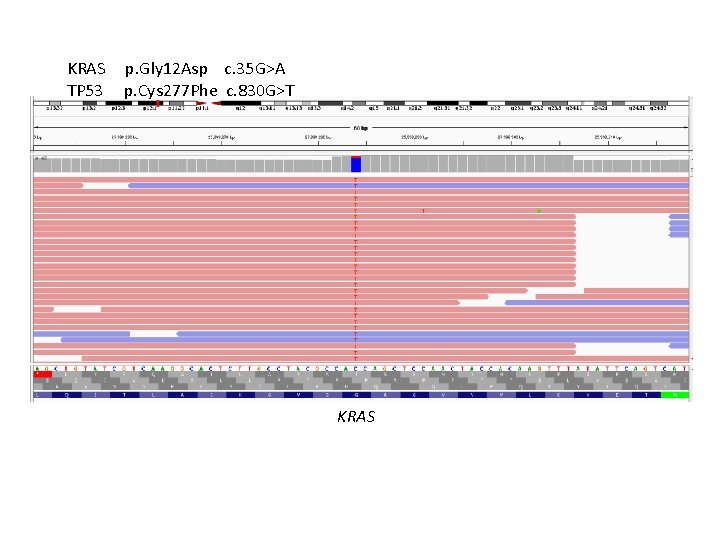
Case Presentation 63 year old female with a 2 year history of recurrent abdominal pain presents to ED in June 2016 with 12 hrs of bilious vomiting. CT reveals a 3 cm pancreatic head mass. Received ERCP and bile duct stenting for symptomatic relief but had 30 lbs weight loss of next two months Biopsy reveals adenocarcinoma Neoadjuvant radiation therapy, follow by resection (3 cm, 4 pos nodes) and chemotherapy Tissue received for molecular analysis….

KRAS TP 53 p. Gly 12 Asp c. 35 G>A p. Cys 277 Phe c. 830 G>T KRAS

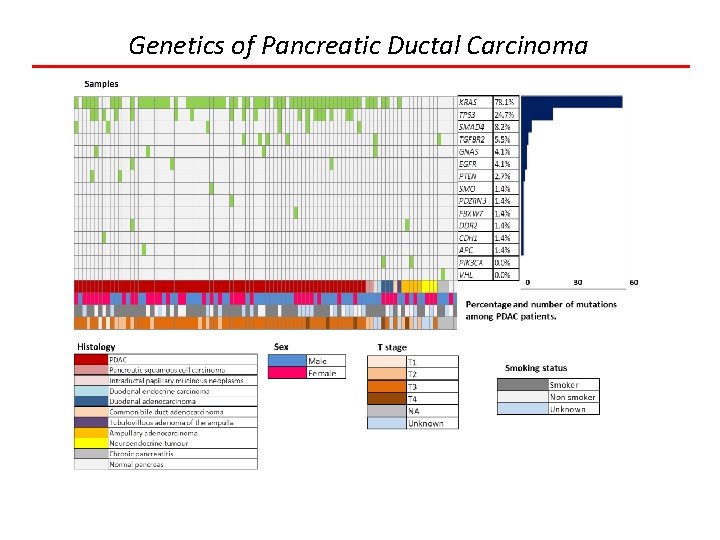
Genetics of Pancreatic Ductal Carcinoma

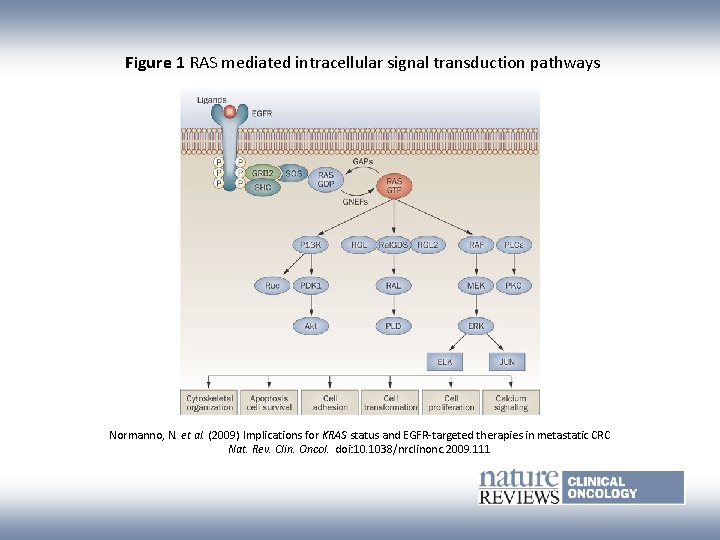
Figure 1 RAS mediated intracellular signal transduction pathways Normanno, N. et al. (2009) Implications for KRAS status and EGFR-targeted therapies in metastatic CRC Nat. Rev. Clin. Oncol. doi: 10. 1038/nrclinonc. 2009. 111

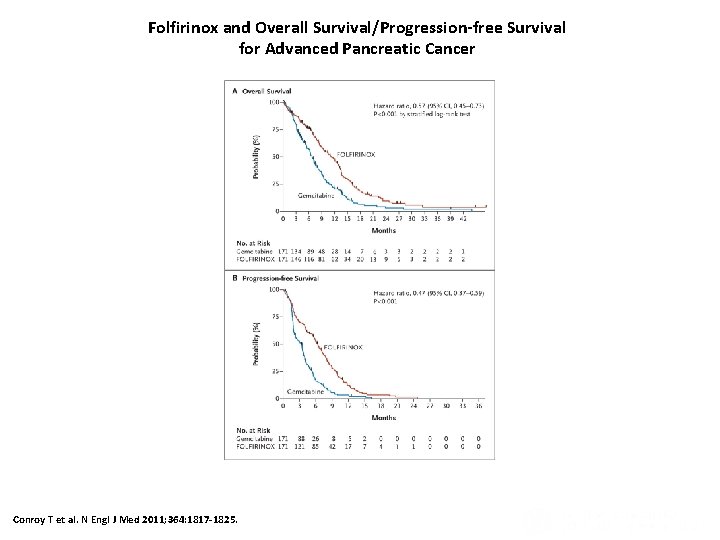
Folfirinox and Overall Survival/Progression-free Survival for Advanced Pancreatic Cancer Conroy T et al. N Engl J Med 2011; 364: 1817 -1825.

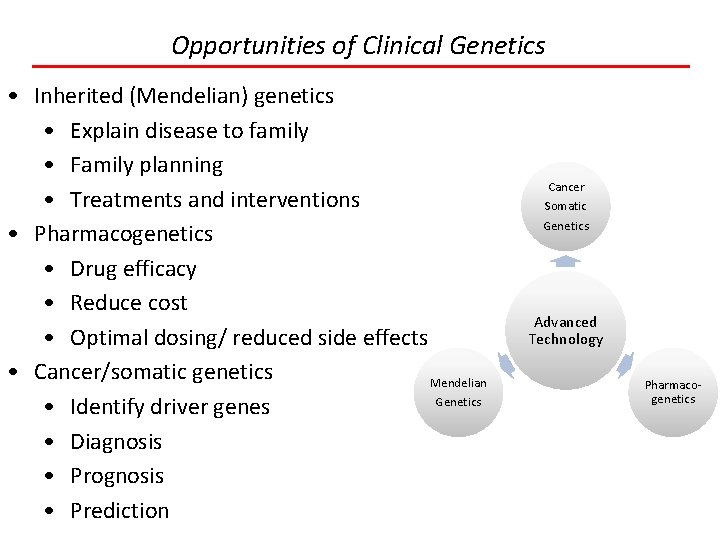
Opportunities of Clinical Genetics • Inherited (Mendelian) genetics • Explain disease to family • Family planning • Treatments and interventions • Pharmacogenetics • Drug efficacy • Reduce cost • Optimal dosing/ reduced side effects • Cancer/somatic genetics Mendelian Genetics • Identify driver genes • Diagnosis • Prognosis • Prediction Cancer Somatic Genetics Advanced Technology Pharmacogenetics

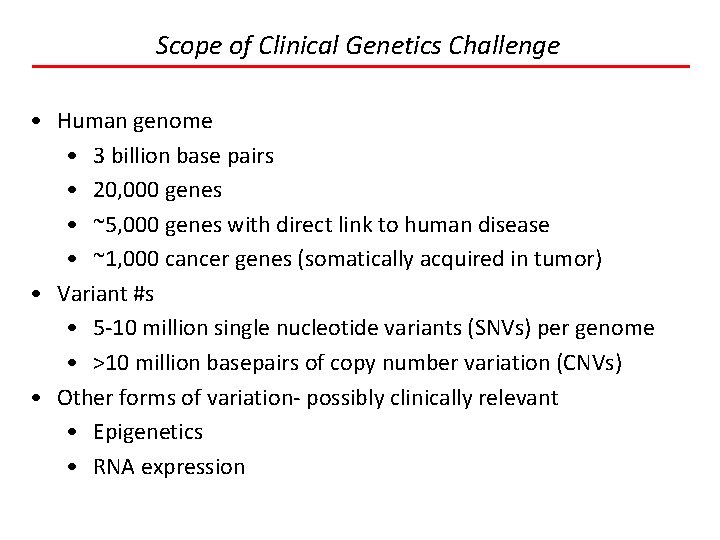
Scope of Clinical Genetics Challenge • Human genome • 3 billion base pairs • 20, 000 genes • ~5, 000 genes with direct link to human disease • ~1, 000 cancer genes (somatically acquired in tumor) • Variant #s • 5 -10 million single nucleotide variants (SNVs) per genome • >10 million basepairs of copy number variation (CNVs) • Other forms of variation- possibly clinically relevant • Epigenetics • RNA expression

Future of Clinical Diagnostics is Here…. Lab automation Information Technology Miniaturization/POCT/Bloodbased Biomarkers Digital imaging Advanced genetics

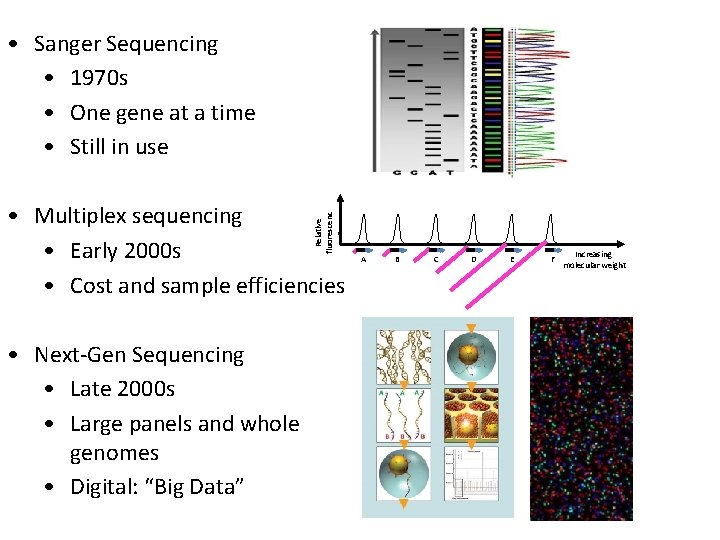
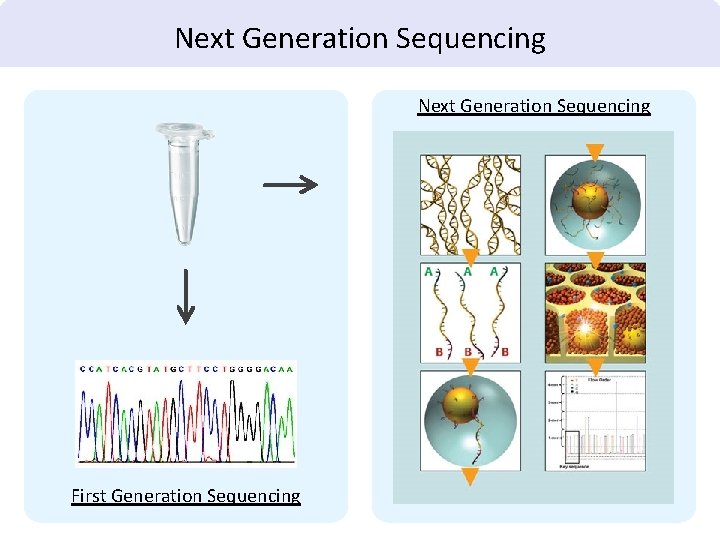
• Sanger Sequencing • 1970 s • One gene at a time • Still in use Relative fluorescenc e • Multiplex sequencing • Early 2000 s • Cost and sample efficiencies • Next-Gen Sequencing • Late 2000 s • Large panels and whole genomes • Digital: “Big Data” A B C D E F Increasing molecular weight

Next Generation Sequencing First Generation Sequencing

Background • NGS has rapidly moved into molecular labs Ø Commercial kits- none FDA approved- all LDTs Ø Commercials labs- numerous: small panels to exomes Ø Academic labs- genomic efforts are largely subsidized or research • Cell-free approaches coming quickly Ø Little performance data Ø Advanced stages • Resistance testing • Who will pay? ?

Challenges in Establishing a Clinical Genotyping Program Strategic • Is this really research? Is this really clinical work? • Universal testing for all tumors? • Financial sustainability and reimbursement Operational • Single gene vs. panels vs. exomes? • Platform and clinical validation • Archived specimen size and quality and turn around time • Informatics Regulatory • Patient consent • FDA ; LDT guidance

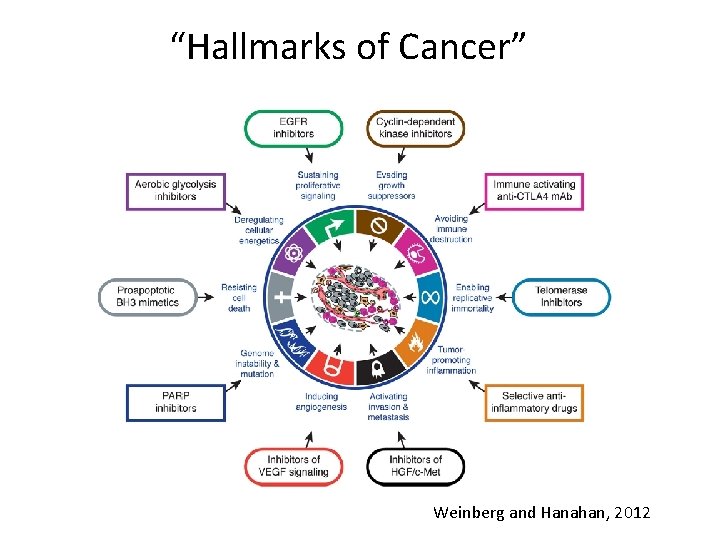
Cancer is a Genetic Disease • Somatic cells accumulate genetic changes – Mutations in DNA – Epigenetic Changes in DNA • Stem cells are target of initial genetic change – Stem cells self renew – Mutation passed on to all progeny – Cancer is a clonal disease • No single mutation cause cancer – Accumulation of mutations give rise to the phenotypic attributes of cancer – Such attributes are called “Hallmarks of Cancer” Courtesy of Dr. Vinay Kumar

Targets of Genetic Damage • Proto-oncogenes: promote cell growth and are dominant in their action ( loss of one normal allele) • Tumor Suppressor genes: inhibit cell growth and are typically recessive ( loss of both alleles) but in some cases loss of one copy ( haploinsufficiency) is enough Courtesy of Dr. Vinay Kumar

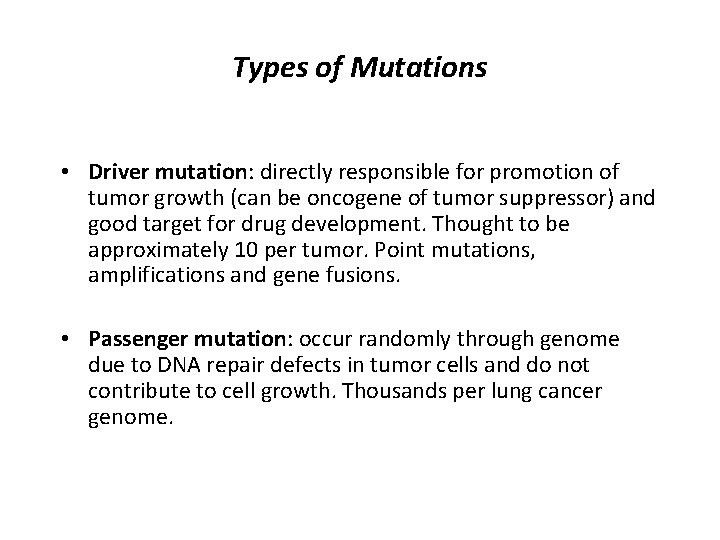
Types of Mutations • Driver mutation: directly responsible for promotion of tumor growth (can be oncogene of tumor suppressor) and good target for drug development. Thought to be approximately 10 per tumor. Point mutations, amplifications and gene fusions. • Passenger mutation: occur randomly through genome due to DNA repair defects in tumor cells and do not contribute to cell growth. Thousands per lung cancer genome.

“Hallmarks of Cancer” Weinberg and Hanahan, 2012

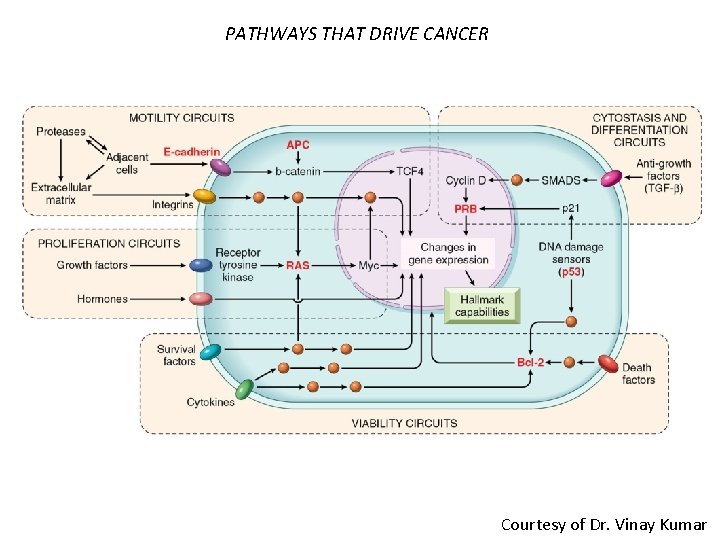
PATHWAYS THAT DRIVE CANCER Courtesy of Dr. Vinay Kumar

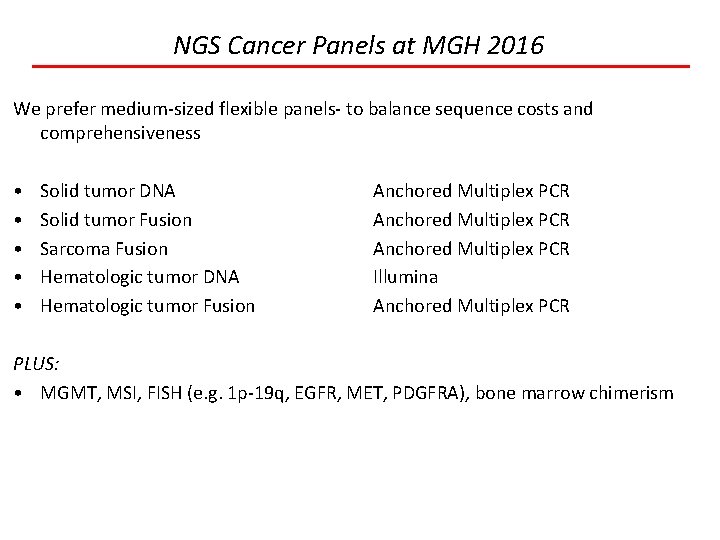
NGS Cancer Panels at MGH 2016 We prefer medium-sized flexible panels- to balance sequence costs and comprehensiveness • • • Solid tumor DNA Solid tumor Fusion Sarcoma Fusion Hematologic tumor DNA Hematologic tumor Fusion Anchored Multiplex PCR Illumina Anchored Multiplex PCR PLUS: • MGMT, MSI, FISH (e. g. 1 p-19 q, EGFR, MET, PDGFRA), bone marrow chimerism

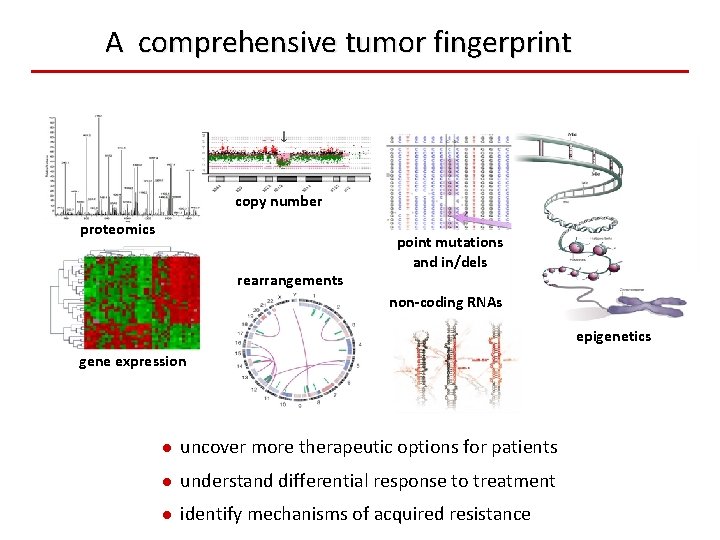
A comprehensive tumor fingerprint copy number proteomics rearrangements point mutations and in/dels non-coding RNAs epigenetics gene expression ● uncover more therapeutic options for patients ● understand differential response to treatment ● identify mechanisms of acquired resistance

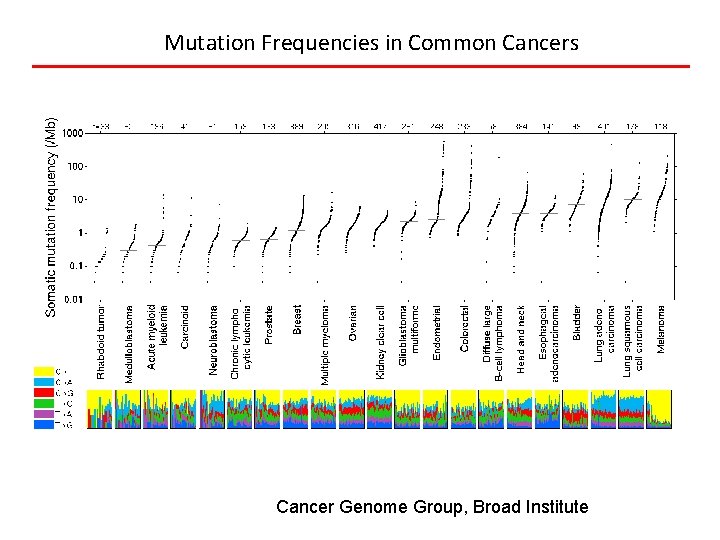
Mutation Frequencies in Common Cancers Cancer Genome Group, Broad Institute

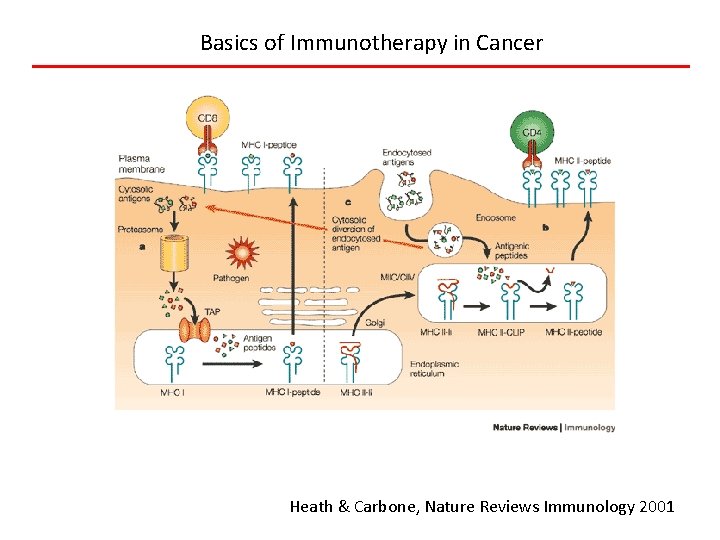
Basics of Immunotherapy in Cancer Heath & Carbone, Nature Reviews Immunology 2001

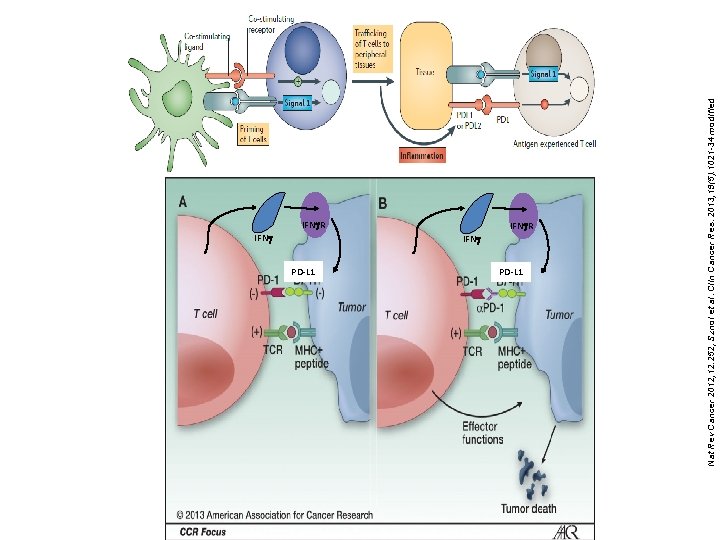
IFNg. R IFNg PD-L 1 IFNg. R PD-L 1 Nat Rev Cancer 2012; 12: 252, Sznol et al. Clin Cancer Res. 2013; 19(5): 1021 -34 modified IFNg

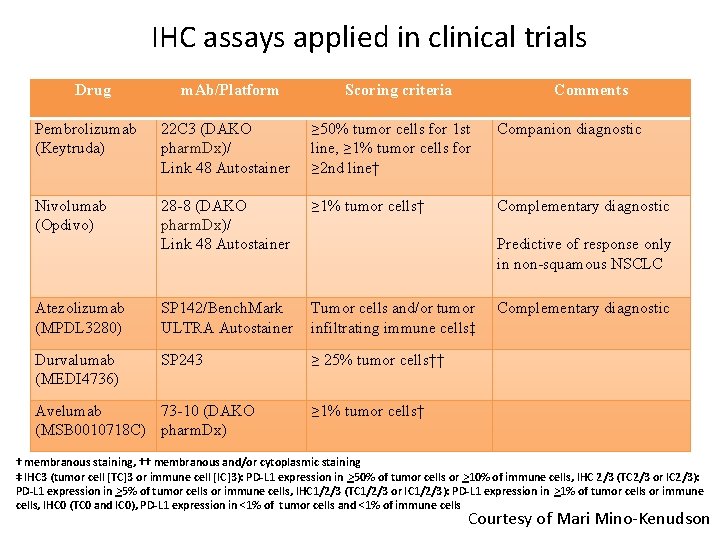
IHC assays applied in clinical trials Drug m. Ab/Platform Scoring criteria Comments Pembrolizumab (Keytruda) 22 C 3 (DAKO pharm. Dx)/ Link 48 Autostainer ≥ 50% tumor cells for 1 st line, ≥ 1% tumor cells for ≥ 2 nd line† Companion diagnostic Nivolumab (Opdivo) 28 -8 (DAKO pharm. Dx)/ Link 48 Autostainer ≥ 1% tumor cells† Complementary diagnostic Predictive of response only in non-squamous NSCLC Atezolizumab (MPDL 3280) SP 142/Bench. Mark ULTRA Autostainer Tumor cells and/or tumor infiltrating immune cells‡ Complementary diagnostic Durvalumab (MEDI 4736) SP 243 ≥ 25% tumor cells†† ≥ 1% tumor cells† Avelumab 73 -10 (DAKO (MSB 0010718 C) pharm. Dx) † membranous staining, †† membranous and/or cytoplasmic staining ‡ IHC 3 (tumor cell [TC]3 or immune cell [IC]3): PD-L 1 expression in >50% of tumor cells or >10% of immune cells, IHC 2/3 (TC 2/3 or IC 2/3): PD-L 1 expression in >5% of tumor cells or immune cells, IHC 1/2/3 (TC 1/2/3 or IC 1/2/3): PD-L 1 expression in >1% of tumor cells or immune cells, IHC 0 (TC 0 and IC 0), PD-L 1 expression in <1% of tumor cells and <1% of immune cells Courtesy of Mari Mino-Kenudson

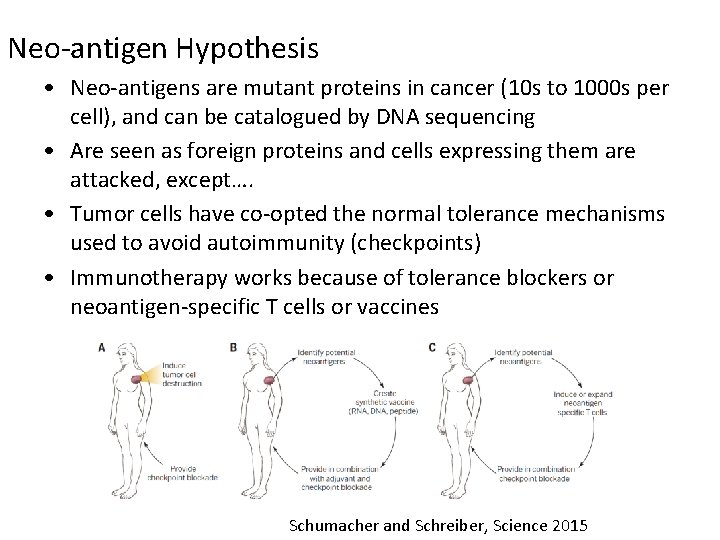
Neo-antigen Hypothesis • Neo-antigens are mutant proteins in cancer (10 s to 1000 s per cell), and can be catalogued by DNA sequencing • Are seen as foreign proteins and cells expressing them are attacked, except…. • Tumor cells have co-opted the normal tolerance mechanisms used to avoid autoimmunity (checkpoints) • Immunotherapy works because of tolerance blockers or neoantigen-specific T cells or vaccines Schumacher and Schreiber, Science 2015

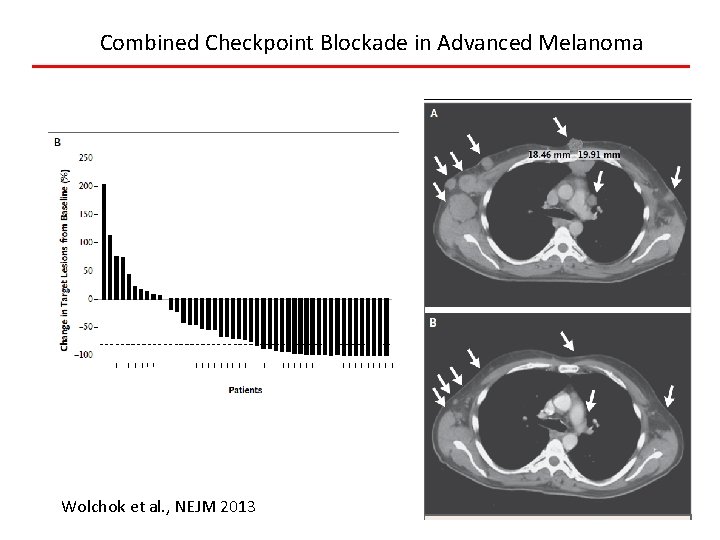
Combined Checkpoint Blockade in Advanced Melanoma Wolchok et al. , NEJM 2013

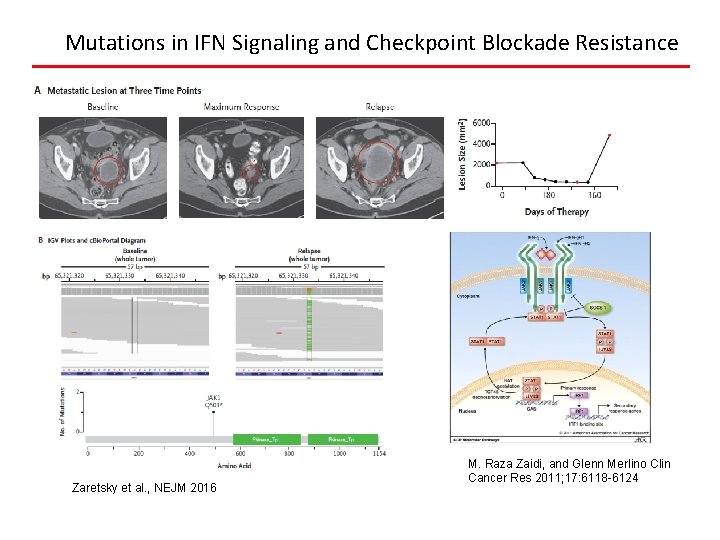
Mutations in IFN Signaling and Checkpoint Blockade Resistance Zaretsky et al. , NEJM 2016 M. Raza Zaidi, and Glenn Merlino Clin Cancer Res 2011; 17: 6118 -6124

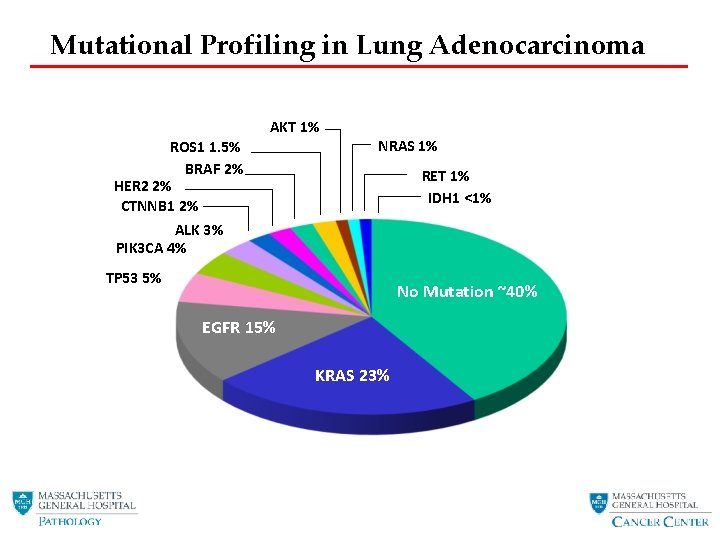
Mutational Profiling in Lung Adenocarcinoma AKT 1% ROS 1 1. 5% BRAF 2% HER 2 2% CTNNB 1 2% ALK 3% PIK 3 CA 4% NRAS 1% RET 1% IDH 1 <1% TP 53 5% No Mutation ~40% EGFR 15% KRAS 23%

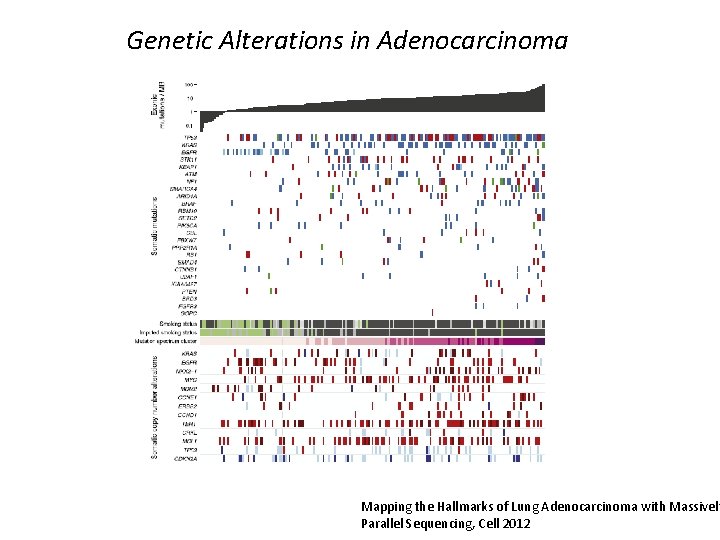
Genetic Alterations in Adenocarcinoma Mapping the Hallmarks of Lung Adenocarcinoma with Massively Parallel Sequencing, Cell 2012

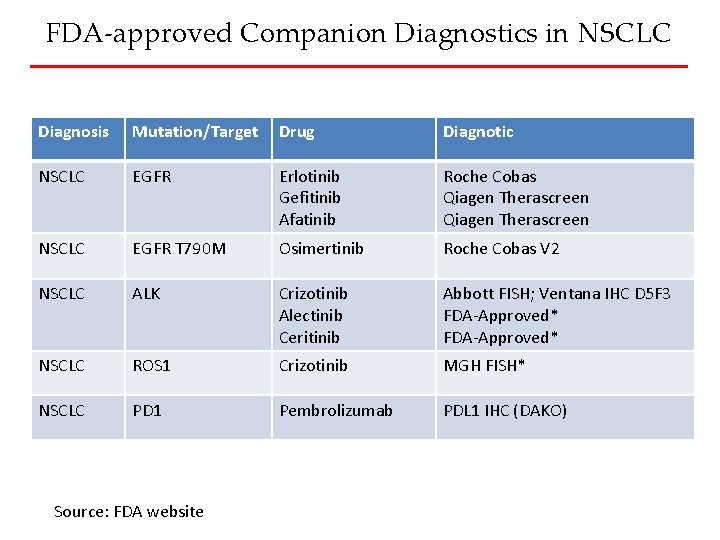
FDA-approved Companion Diagnostics in NSCLC Diagnosis Mutation/Target Drug Diagnotic NSCLC EGFR Erlotinib Gefitinib Afatinib Roche Cobas Qiagen Therascreen NSCLC EGFR T 790 M Osimertinib Roche Cobas V 2 NSCLC ALK Crizotinib Alectinib Ceritinib Abbott FISH; Ventana IHC D 5 F 3 FDA-Approved* NSCLC ROS 1 Crizotinib MGH FISH* NSCLC PD 1 Pembrolizumab PDL 1 IHC (DAKO) Source: FDA website

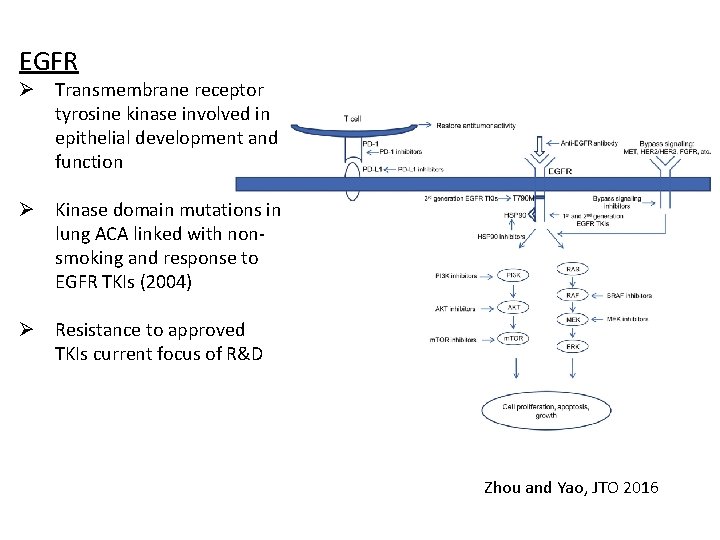
EGFR Ø Transmembrane receptor tyrosine kinase involved in epithelial development and function Ø Kinase domain mutations in lung ACA linked with nonsmoking and response to EGFR TKIs (2004) Ø Resistance to approved TKIs current focus of R&D Zhou and Yao, JTO 2016

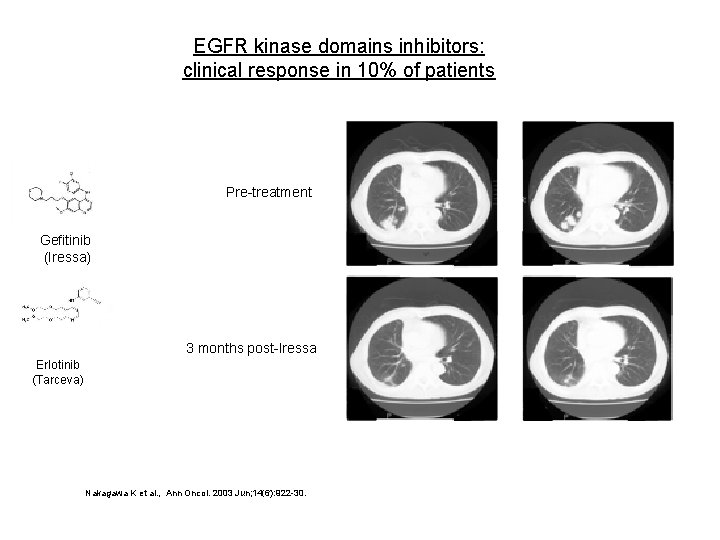
EGFR kinase domains inhibitors: clinical response in 10% of patients Pre-treatment Gefitinib (Iressa) 3 months post-Iressa Erlotinib (Tarceva) Nakagawa K et al. , Ann Oncol. 2003 Jun; 14(6): 922 -30.

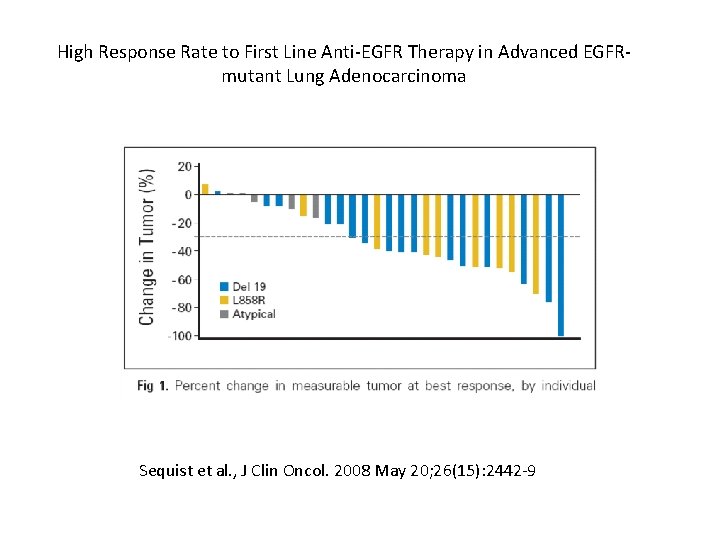
High Response Rate to First Line Anti-EGFR Therapy in Advanced EGFRmutant Lung Adenocarcinoma Sequist et al. , J Clin Oncol. 2008 May 20; 26(15): 2442 -9

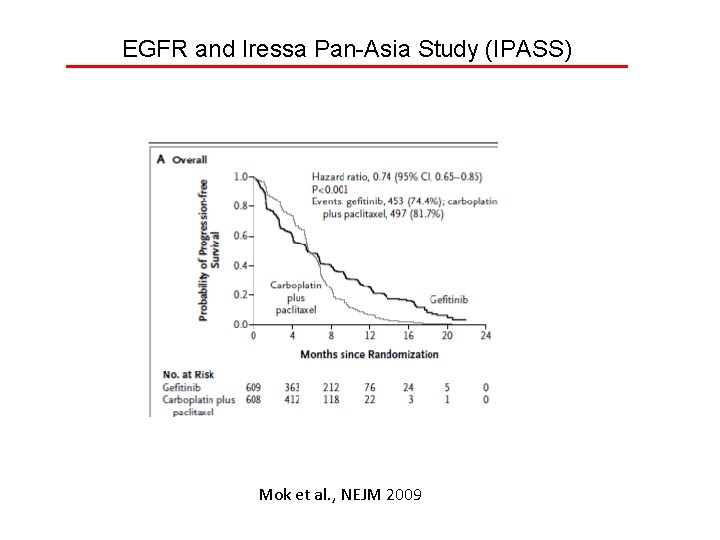
EGFR and Iressa Pan-Asia Study (IPASS) Mok et al. , NEJM 2009

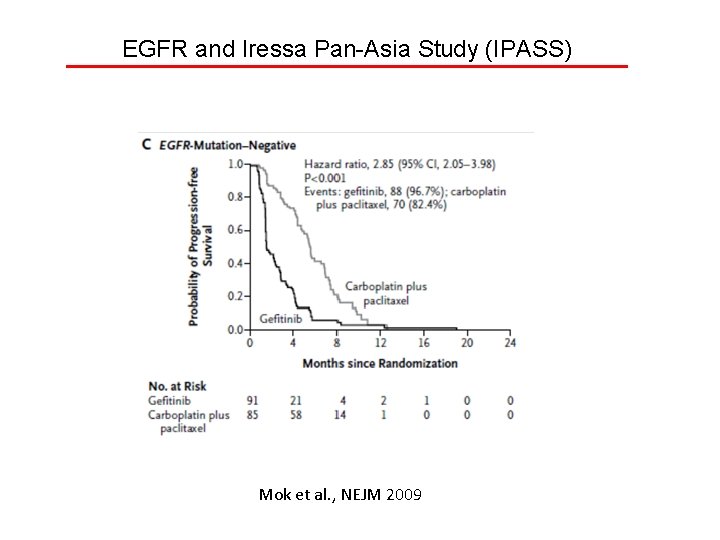
EGFR and Iressa Pan-Asia Study (IPASS) Mok et al. , NEJM 2009

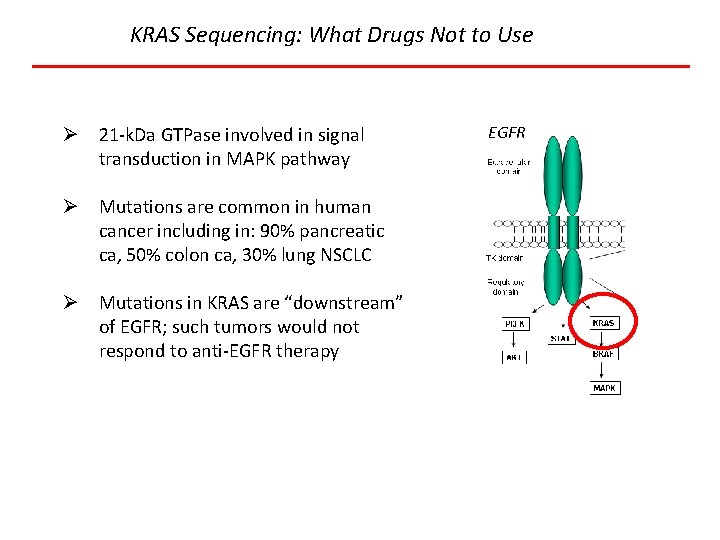
KRAS Sequencing: What Drugs Not to Use Ø 21 -k. Da GTPase involved in signal transduction in MAPK pathway Ø Mutations are common in human cancer including in: 90% pancreatic ca, 50% colon ca, 30% lung NSCLC Ø Mutations in KRAS are “downstream” of EGFR; such tumors would not respond to anti-EGFR therapy EGFR

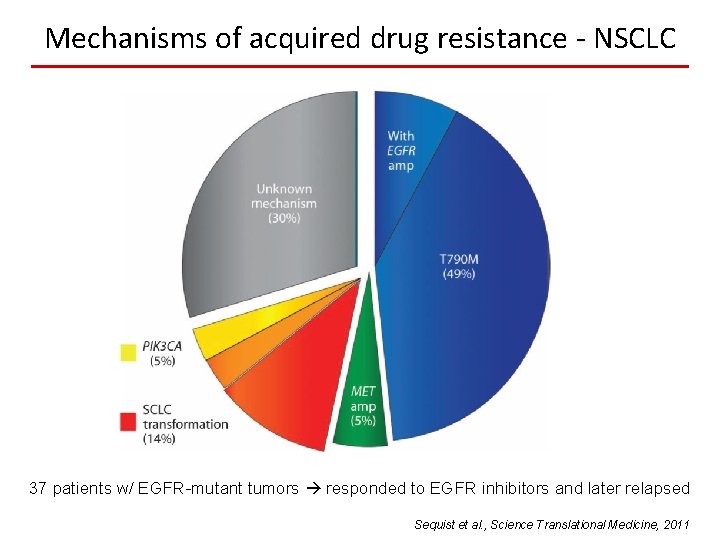
Mechanisms of acquired drug resistance - NSCLC 37 patients w/ EGFR-mutant tumors responded to EGFR inhibitors and later relapsed Sequist et al. , Science Translational Medicine, 2011

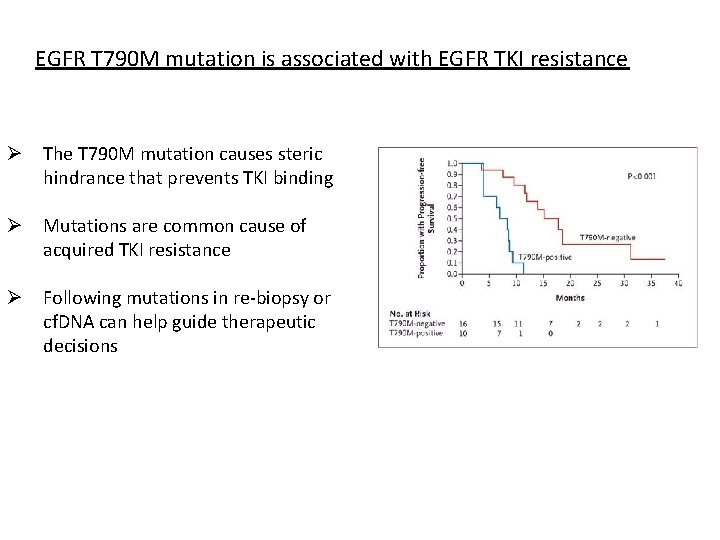
EGFR T 790 M mutation is associated with EGFR TKI resistance Ø The T 790 M mutation causes steric hindrance that prevents TKI binding Ø Mutations are common cause of acquired TKI resistance Ø Following mutations in re-biopsy or cf. DNA can help guide therapeutic decisions

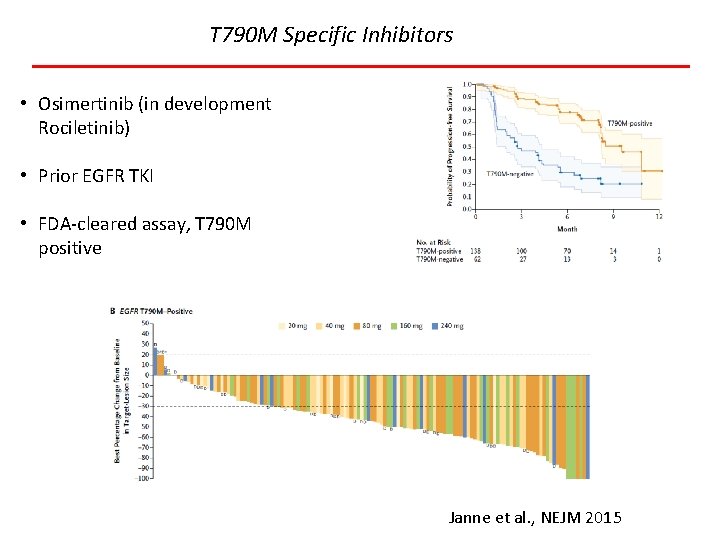
T 790 M Specific Inhibitors • Osimertinib (in development Rociletinib) • Prior EGFR TKI • FDA-cleared assay, T 790 M positive Janne et al. , NEJM 2015

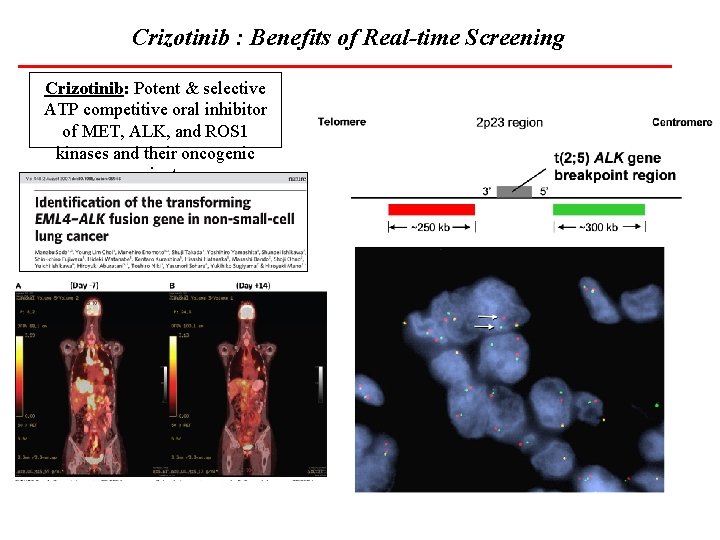
Crizotinib : Benefits of Real-time Screening Crizotinib: Potent & selective ATP competitive oral inhibitor of MET, ALK, and ROS 1 kinases and their oncogenic variants

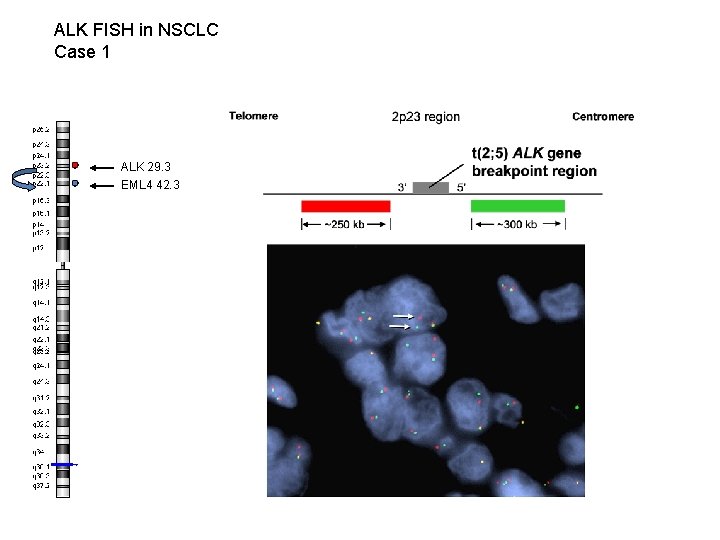
ALK FISH in NSCLC Case 1 ALK 29. 3 EML 4 42. 3 WT (non-split) signal Split signal

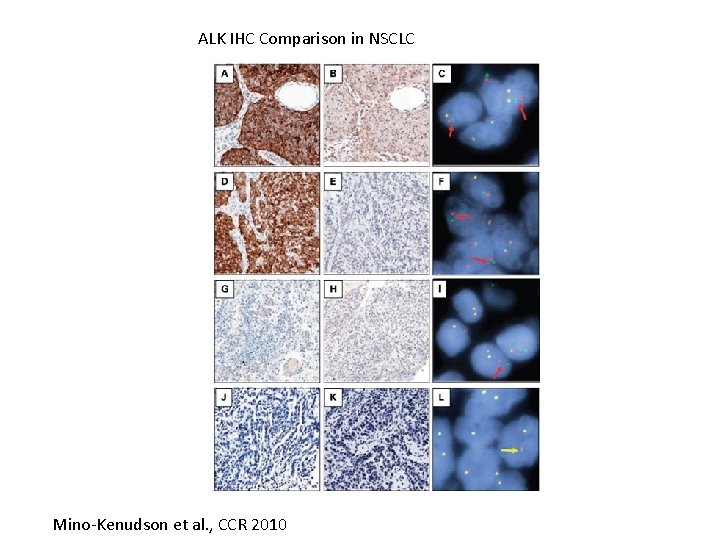
ALK IHC Comparison in NSCLC Mino-Kenudson et al. , CCR 2010

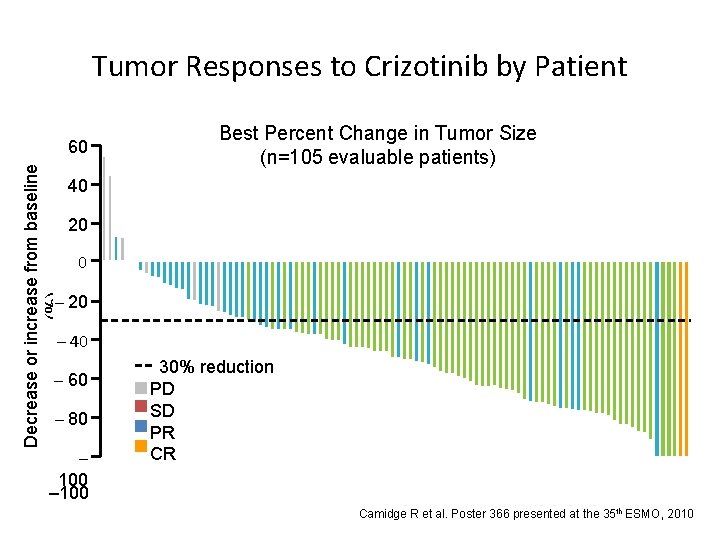
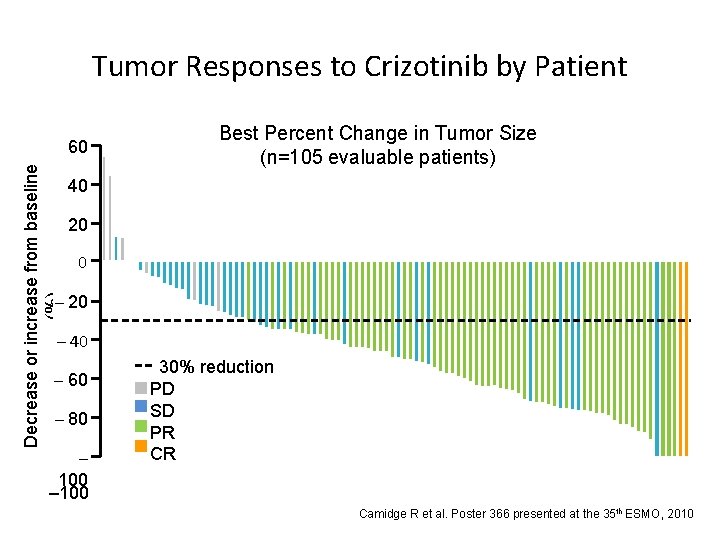
Decrease or increase from baseline (%) Tumor Responses to Crizotinib by Patient 60 50 40 Best Percent Change in Tumor Size (n=105 evaluable patients) 20 20 0 –– 10 20 – 40 – 60 –– 70 80 – 100 30% reduction PD SD PR CR Camidge R et al. Poster 366 presented at the 35 th ESMO, 2010

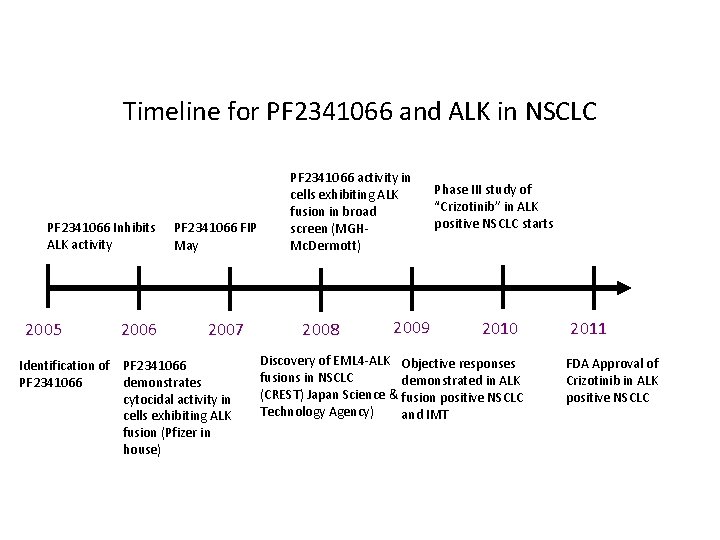
Timeline for PF 2341066 and ALK in NSCLC PF 2341066 Inhibits ALK activity 2005 2006 PF 2341066 FIP May 2007 Identification of PF 2341066 demonstrates cytocidal activity in cells exhibiting ALK fusion (Pfizer in house) PF 2341066 activity in cells exhibiting ALK fusion in broad screen (MGHMc. Dermott) 2008 2009 Phase III study of “Crizotinib” in ALK positive NSCLC starts 2010 Discovery of EML 4 -ALK Objective responses fusions in NSCLC demonstrated in ALK (CREST) Japan Science & fusion positive NSCLC Technology Agency) and IMT 2011 FDA Approval of Crizotinib in ALK positive NSCLC

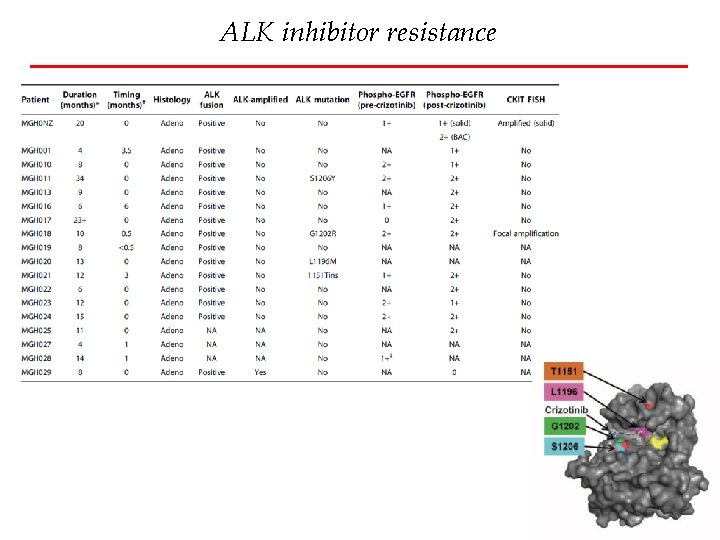
ALK inhibitor resistance

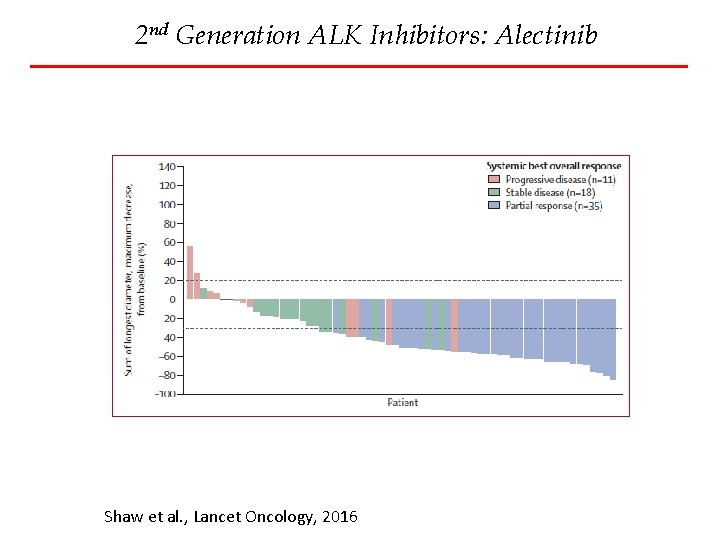
2 nd Generation ALK Inhibitors: Alectinib Shaw et al. , Lancet Oncology, 2016

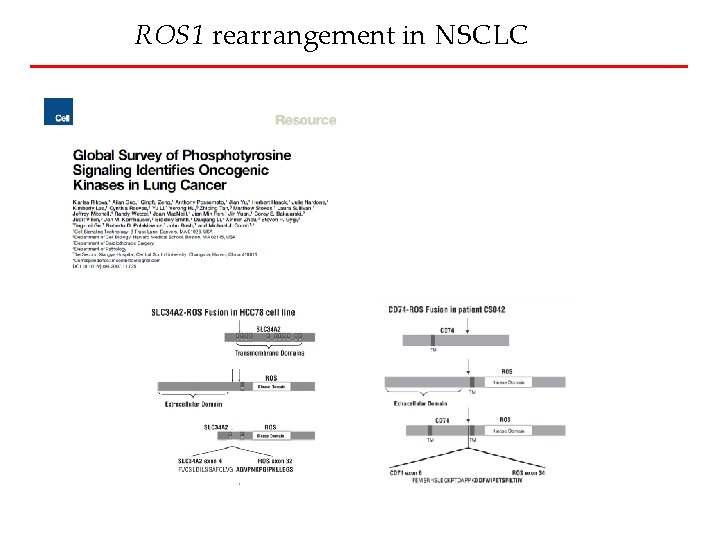
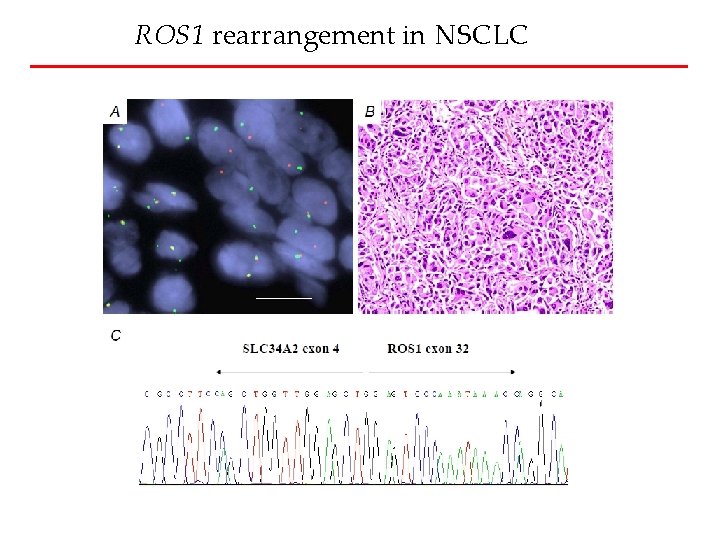
ROS 1 rearrangement in NSCLC

ROS 1 rearrangement in NSCLC

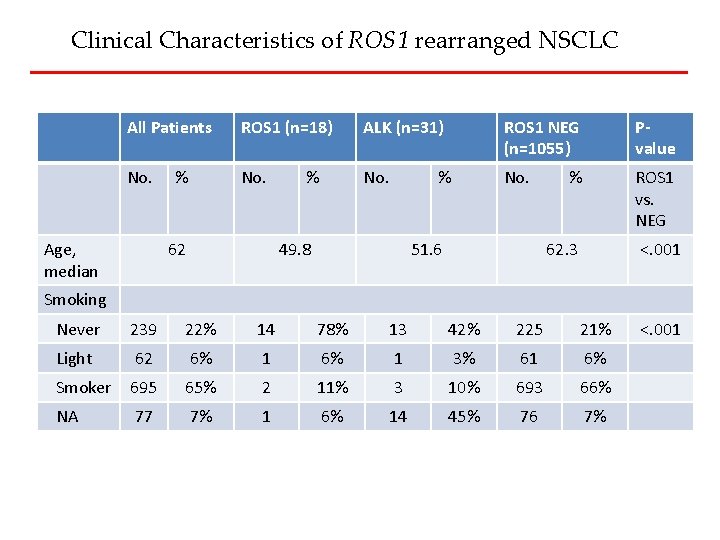
Clinical Characteristics of ROS 1 rearranged NSCLC All Patients ROS 1 (n=18) ALK (n=31) ROS 1 NEG (n=1055) Pvalue No. ROS 1 vs. NEG Age, median % 62 % % 49. 8 51. 6 % 62. 3 <. 001 Smoking Never 239 22% 14 78% 13 42% 225 21% Light 62 6% 1 3% 61 6% Smoker 695 65% 2 11% 3 10% 693 66% NA 77 7% 1 6% 14 45% 76 7% <. 001

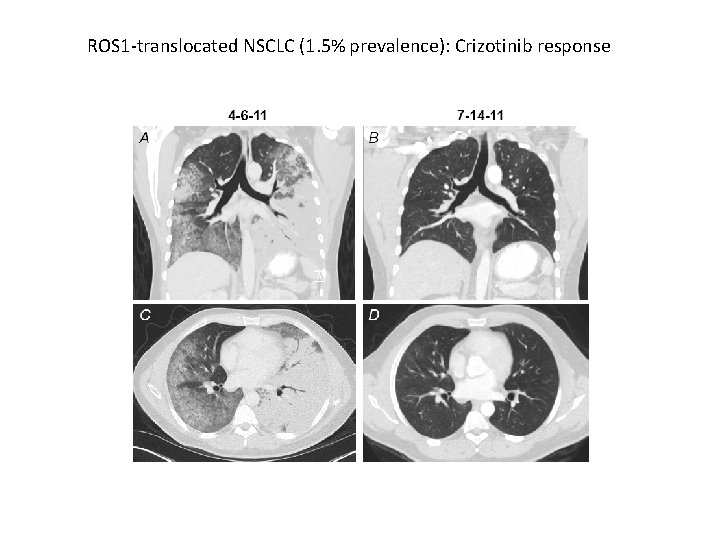
ROS 1 -translocated NSCLC (1. 5% prevalence): Crizotinib response

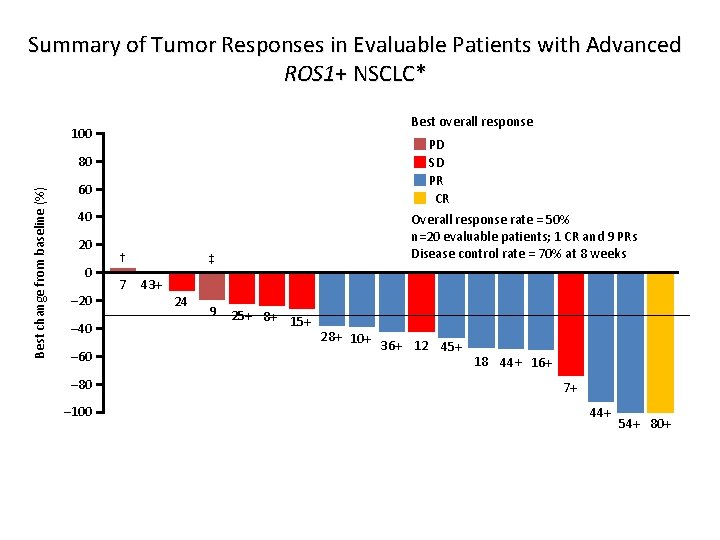
Summary of Tumor Responses in Evaluable Patients with Advanced ROS 1+ NSCLC* Best overall response 100 PD SD PR CR Best change from baseline (%) 80 60 40 20 0 – 20 – 40 – 60 – 80 – 100 † 7 Overall response rate = 50% n=20 evaluable patients; 1 CR and 9 PRs Disease control rate = 70% at 8 weeks ‡ 43+ 24 9 25+ 8+ 15+ 28+ 10+ 36+ 12 45+ 18 44+ 16+ 7+ 44+ 54+ 80+

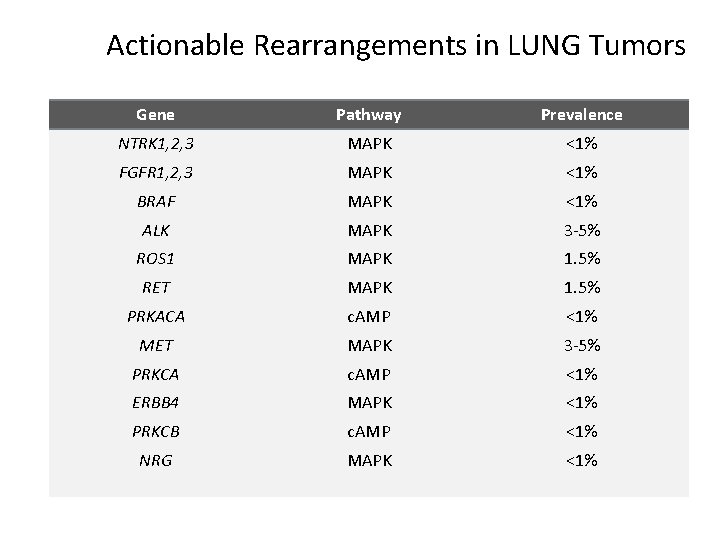
Actionable Rearrangements in LUNG Tumors Gene Pathway Prevalence NTRK 1, 2, 3 MAPK <1% FGFR 1, 2, 3 MAPK <1% BRAF MAPK <1% ALK MAPK 3 -5% ROS 1 MAPK 1. 5% RET MAPK 1. 5% PRKACA c. AMP <1% MET MAPK 3 -5% PRKCA c. AMP <1% ERBB 4 MAPK <1% PRKCB c. AMP <1% NRG MAPK <1%

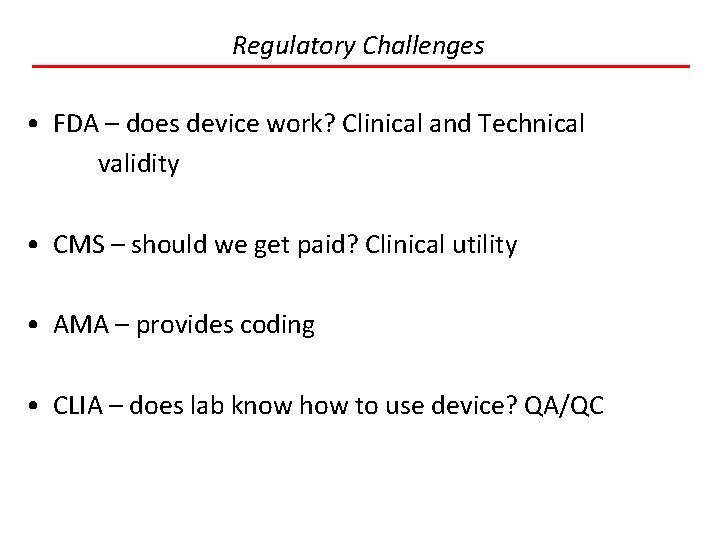
Regulatory Challenges • FDA – does device work? Clinical and Technical validity • CMS – should we get paid? Clinical utility • AMA – provides coding • CLIA – does lab know how to use device? QA/QC

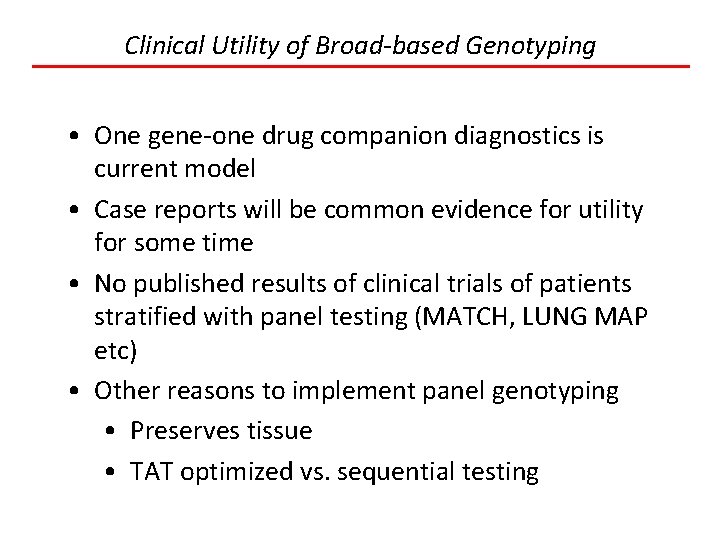
Clinical Utility of Broad-based Genotyping • One gene-one drug companion diagnostics is current model • Case reports will be common evidence for utility for some time • No published results of clinical trials of patients stratified with panel testing (MATCH, LUNG MAP etc) • Other reasons to implement panel genotyping • Preserves tissue • TAT optimized vs. sequential testing

Decrease or increase from baseline (%) Tumor Responses to Crizotinib by Patient 60 50 40 Best Percent Change in Tumor Size (n=105 evaluable patients) 20 20 0 –– 10 20 – 40 – 60 –– 70 80 – 100 30% reduction PD SD PR CR Camidge R et al. Poster 366 presented at the 35 th ESMO, 2010

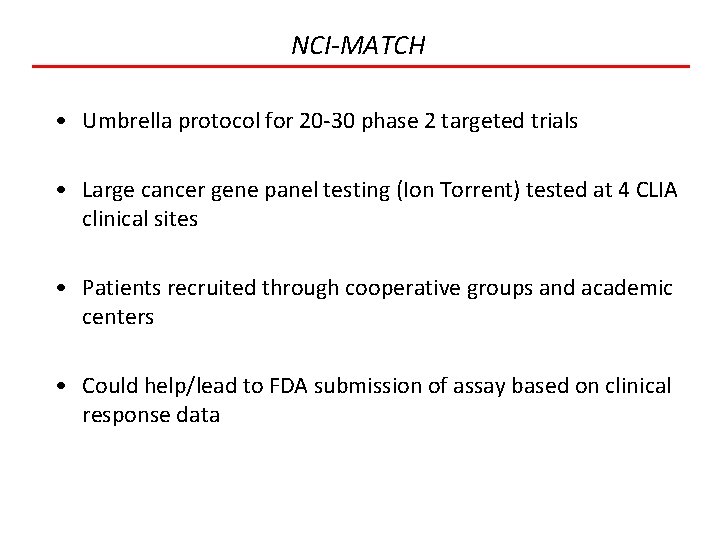
NCI-MATCH • Umbrella protocol for 20 -30 phase 2 targeted trials • Large cancer gene panel testing (Ion Torrent) tested at 4 CLIA clinical sites • Patients recruited through cooperative groups and academic centers • Could help/lead to FDA submission of assay based on clinical response data

NGS MAC NGS Program • NSCLC panel policy proposal for 81445 – made with input from 5 academic medical centers • Newly diagnosed NSCLC patients with advanced (stage IIIB or IV) NSCLC, who are not treatable by resection or radiation with curative intent, and who are suitable candidates for therapy at the time of testing. • No smoking related rules • No comment on prior testing • No test quality standards

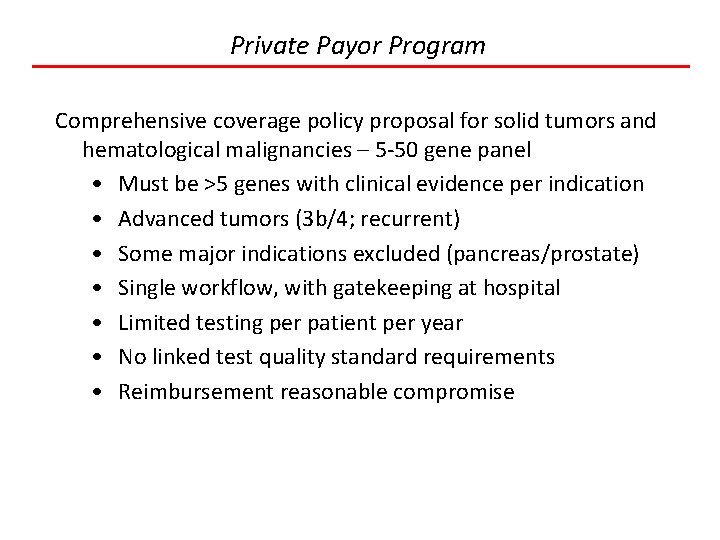
Private Payor Program Comprehensive coverage policy proposal for solid tumors and hematological malignancies – 5 -50 gene panel • Must be >5 genes with clinical evidence per indication • Advanced tumors (3 b/4; recurrent) • Some major indications excluded (pancreas/prostate) • Single workflow, with gatekeeping at hospital • Limited testing per patient per year • No linked test quality standard requirements • Reimbursement reasonable compromise

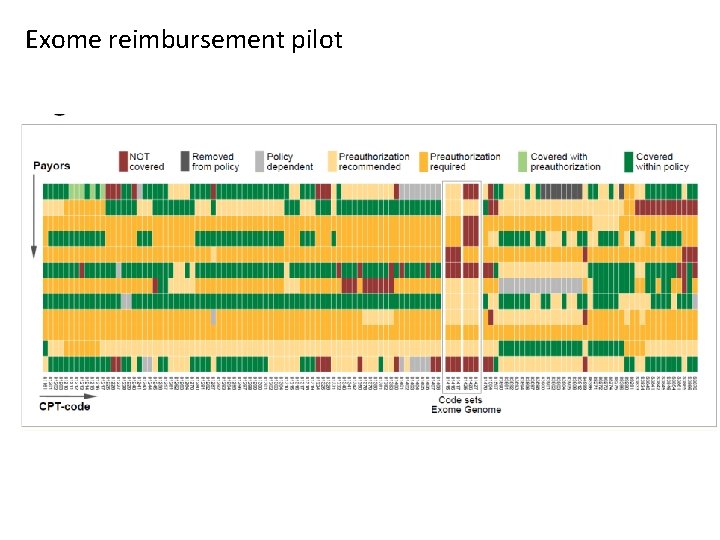
Exome reimbursement pilot

Payor needs • Control costs • Education and medical knowledge • Develop policies that can be audited Ø Currently no trials showing benefit of NGS Ø Almost all other policies have firm evidence based medicine standards

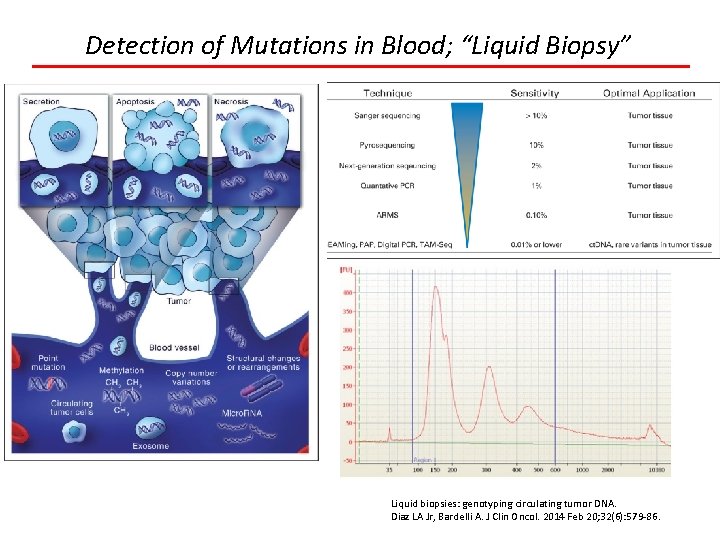
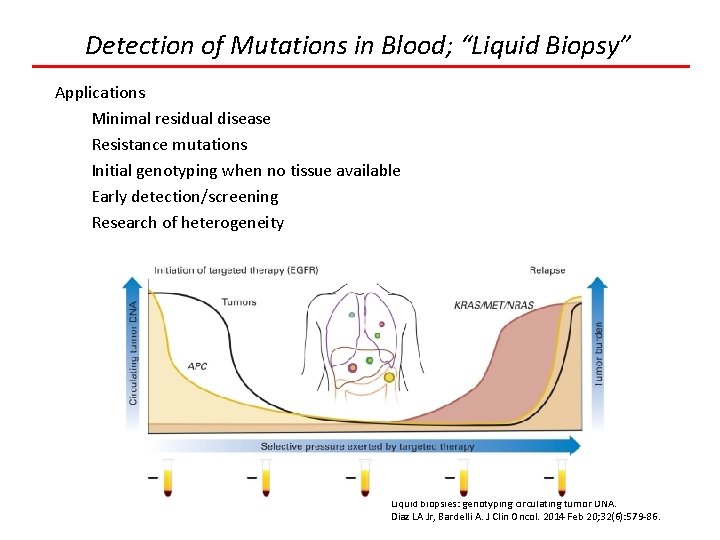
Detection of Mutations in Blood; “Liquid Biopsy” Liquid biopsies: genotyping circulating tumor DNA. Diaz LA Jr, Bardelli A. J Clin Oncol. 2014 Feb 20; 32(6): 579 -86.

Detection of Mutations in Blood; “Liquid Biopsy” Applications Minimal residual disease Resistance mutations Initial genotyping when no tissue available Early detection/screening Research of heterogeneity Liquid biopsies: genotyping circulating tumor DNA. Diaz LA Jr, Bardelli A. J Clin Oncol. 2014 Feb 20; 32(6): 579 -86.

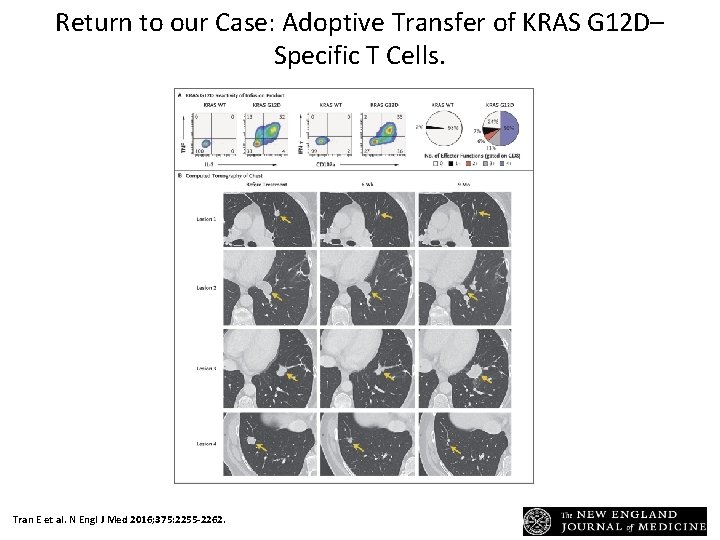
Return to our Case: Adoptive Transfer of KRAS G 12 D– Specific T Cells. Tran E et al. N Engl J Med 2016; 375: 2255 -2262.

Summary • Comprehensive genotyping is here to stay • Critical to our understand of drug response and resistance • Technology continues to move very fast • Need clinical utility evidence and regulatory clarity

Center for Integrated Diagnostics Marianne Boswell Dora Dias-Santagata Long Phi Le Jochen Lennerz Darrell R. Borger Leif Ellisen A. Bernard Collins Kathy Vernovsky Karima Ricketts David Louis Daniel Haber Gaddy Getz Martin Aryee Hayley Robinson Zongli Zheng Julie Batten Valentina Nardi Miguel Rivera
 Giant molecular structure vs simple molecular structure
Giant molecular structure vs simple molecular structure Giant molecular structure vs simple molecular structure
Giant molecular structure vs simple molecular structure Covalent bond melting point
Covalent bond melting point American epilepsy society annual meeting 2017
American epilepsy society annual meeting 2017 8 unique features of e-commerce technology
8 unique features of e-commerce technology Gertler econ
Gertler econ Marlene sallo
Marlene sallo Thomas barnard sermon massachusetts 1763
Thomas barnard sermon massachusetts 1763 Hdmaster massachusetts
Hdmaster massachusetts Project impact massachusetts
Project impact massachusetts Pearsonaccessnext mcas
Pearsonaccessnext mcas Restraint training massachusetts
Restraint training massachusetts Massachusetts timesheet
Massachusetts timesheet Hdap massachusetts
Hdap massachusetts Massachusetts work based learning plan
Massachusetts work based learning plan Adrc massachusetts
Adrc massachusetts Massachusetts bay colony
Massachusetts bay colony Massachusetts food code
Massachusetts food code English calvinists who sought a thorough cleansing
English calvinists who sought a thorough cleansing Efile massachusetts appeals court
Efile massachusetts appeals court Plep a vs plep b
Plep a vs plep b Massachusetts educational financing authority
Massachusetts educational financing authority Massachusetts department of transportation
Massachusetts department of transportation Closed loop geothermal well massachusetts
Closed loop geothermal well massachusetts Massachusetts office of travel & tourism
Massachusetts office of travel & tourism Massachusetts association for professional law enforcement
Massachusetts association for professional law enforcement Danny townsend massachusetts
Danny townsend massachusetts Ma smart program overview
Ma smart program overview Massachusetts renewable portfolio standard
Massachusetts renewable portfolio standard 6176503557
6176503557 Dsrip massachusetts
Dsrip massachusetts Interior points
Interior points Massachusetts higher education consortium
Massachusetts higher education consortium Child poverty in massachusetts
Child poverty in massachusetts Famous landmarks in massachusetts
Famous landmarks in massachusetts Cfma massachusetts
Cfma massachusetts Meaning of massachusetts
Meaning of massachusetts Cvte massachusetts
Cvte massachusetts Philip koziolek
Philip koziolek Rural development loan massachusetts
Rural development loan massachusetts Massachusetts pay transparency law
Massachusetts pay transparency law Massachusetts comprehensive assessment system
Massachusetts comprehensive assessment system Massachusetts v. sheppard
Massachusetts v. sheppard Concept paper example
Concept paper example Data privacy massachusetts
Data privacy massachusetts Tmu map test
Tmu map test Chapter 766 massachusetts law
Chapter 766 massachusetts law Joseph smith salem massachusetts
Joseph smith salem massachusetts Mandated reporting massachusetts
Mandated reporting massachusetts University of massachusetts building authority
University of massachusetts building authority Província da baía de massachusetts
Província da baía de massachusetts Hcsis massachusetts
Hcsis massachusetts Pathology
Pathology Oral pathology
Oral pathology Data-centric pathology
Data-centric pathology Oral pathology
Oral pathology Musculoskeletal
Musculoskeletal Ajkd atlas of renal pathology
Ajkd atlas of renal pathology What pathology
What pathology Gross pathology
Gross pathology Pathology
Pathology Prostate pathology
Prostate pathology Pathology visions
Pathology visions Virtual pathology at the university of leeds
Virtual pathology at the university of leeds In pathology
In pathology Monomorphic adenoma salivary gland
Monomorphic adenoma salivary gland