Massachusetts Department of Public Health e Prescribing Regulations

Massachusetts Department of Public Health e. Prescribing Regulations 105 CMR 721. 000 Standards for Prescription Format and Security in Massachusetts Medical Society December 11, 2019 Lauren B. Nelson, Esq. Director of Policy and Regulatory Affairs Bureau of Health Professions Licensure

Learning Objectives Upon completion of the activity, participants should be better able to: • Identify valid written and oral prescriptions; • Recognize different follow-up and reporting requirements • Classify applicable e. Prescribing exceptions; • Distinguish controlled substance schedule-based dispensing requirements; and • Exercise appropriate discretion when dispensing non-electronic prescriptions. Massachusetts Department of Public Health mass. gov/dph
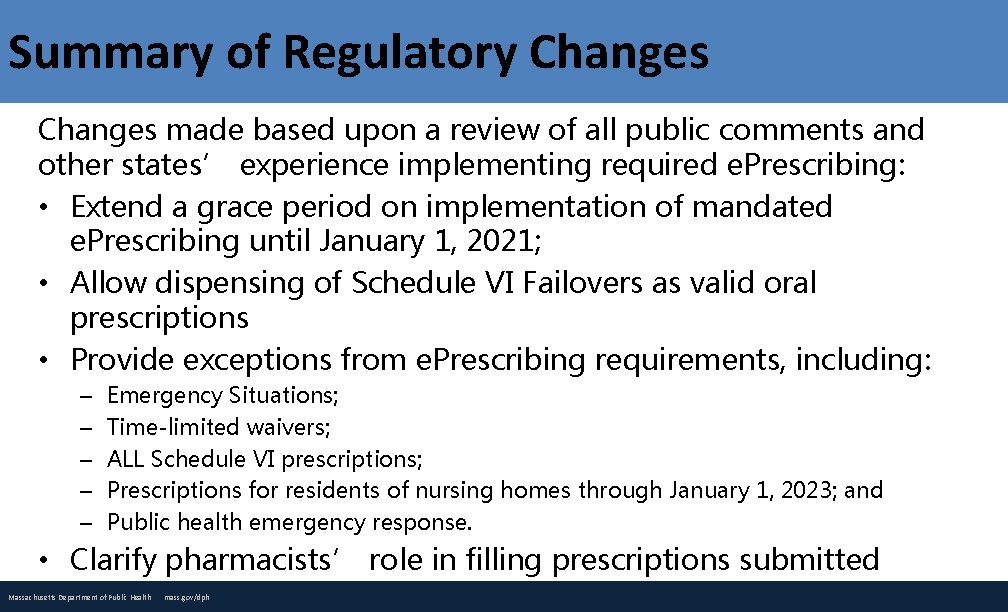
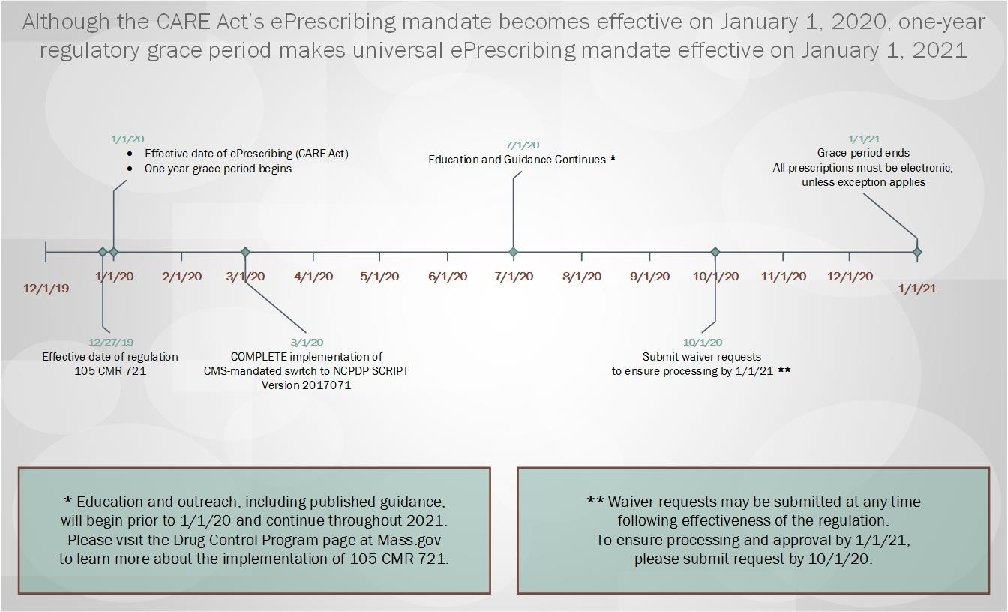
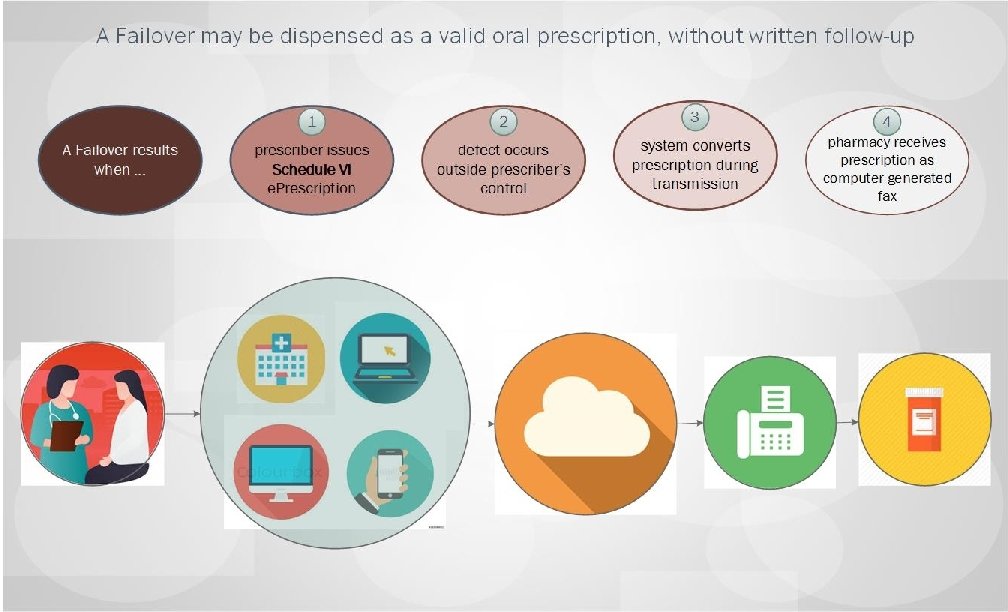
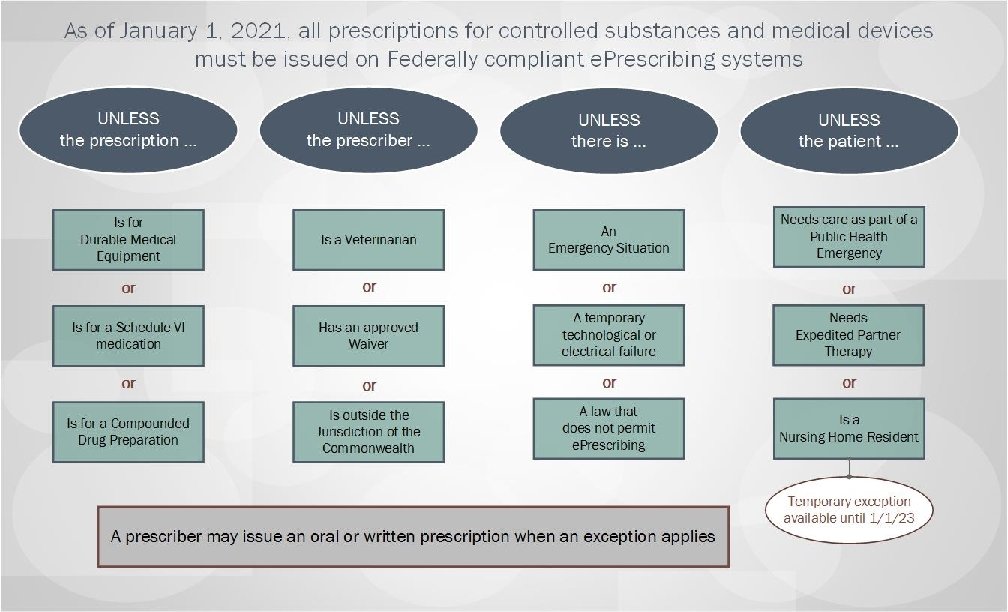
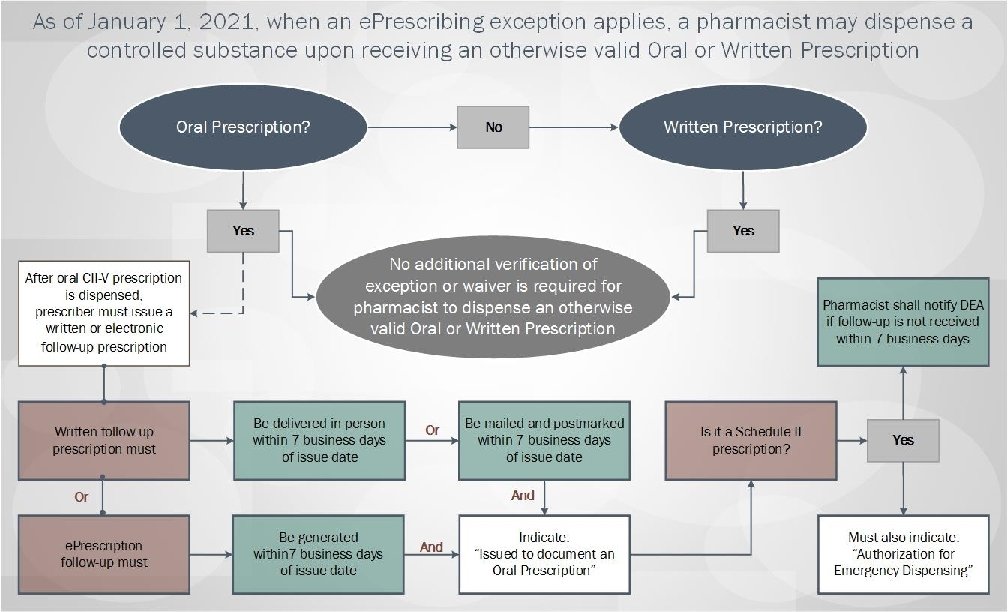
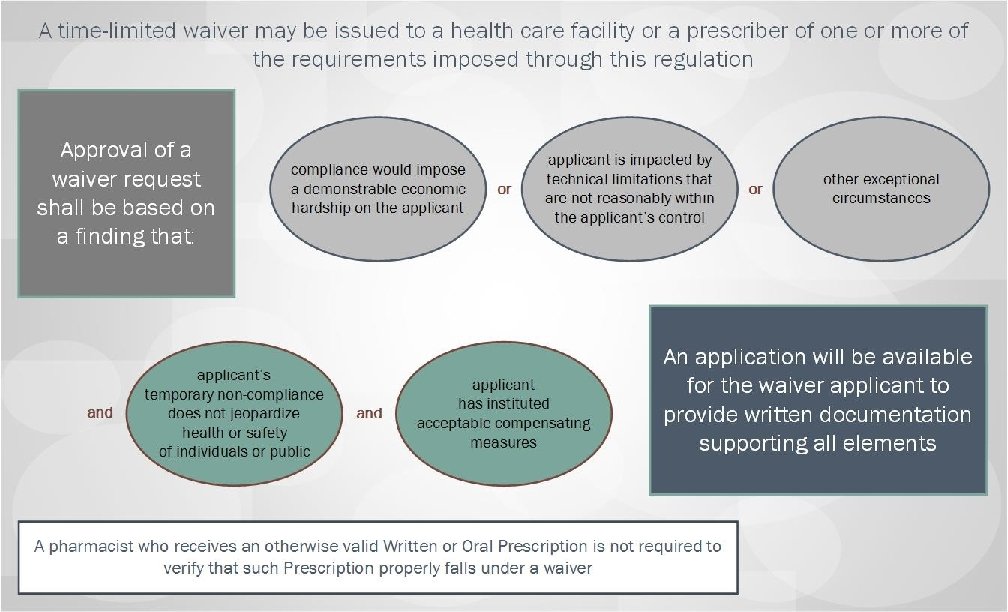
Summary of Regulatory Changes made based upon a review of all public comments and other states’ experience implementing required e. Prescribing: • Extend a grace period on implementation of mandated e. Prescribing until January 1, 2021; • Allow dispensing of Schedule VI Failovers as valid oral prescriptions • Provide exceptions from e. Prescribing requirements, including: – – – Emergency Situations; Time-limited waivers; ALL Schedule VI prescriptions; Prescriptions for residents of nursing homes through January 1, 2023; and Public health emergency response. • Clarify pharmacists’ role in filling prescriptions submitted under an exception or waiver. Massachusetts Department of Public Health mass. gov/dph

Controlled Substance Schedules • • Controlled Substances are placed in a Federal schedule based on – currently accepted medical use in treatment in the United States, – relative abuse potential, and – likelihood of causing dependence when abused. Schedule I Controlled Substances have no currently accepted medical use in the United States, a lack of accepted safety for use under medical supervision, and a high potential for abuse. Examples: heroin, LSD, peyote, & Ecstasy. Schedule II Controlled Substances have a high potential for abuse which may lead to severe psychological or physical dependence. Examples: most opioids and stimulants like Adderall® & Ritalin®. Schedule III Controlled Substances have a lesser potential for abuse than Schedules I or II, and abuse may lead to moderate or low physical dependence or high psychological dependence. Examples: buprenorphine, ketamine & anabolic steroids Schedule IV Controlled Substances have a lower potential for abuse than Schedule III. Examples: Xanax®, Valium® & Ativan® Schedule V Controlled Substances have a lower potential for abuse than Schedule IV and consist primarily of preparations containing limited quantities of certain narcotics. Examples: Robitussin AC®, and Phenergan with Codeine®. Schedule VI Controlled Substances (in Massachusetts) include all other prescription drugs that are not included in Schedules I-V. Examples: antibiotics , naloxone, sterile saline, chemotherapy treatments, oxygen and epi-pens. Massachusetts Department of Public Health mass. gov/dph


Massachusetts Department of Public Health Thank you for the opportunity to present this information today. For more information regarding prescription format and security, please find the relevant statutory language and approved, post-comment regulation here: https: //malegislature. gov/Laws/General. Laws/Part. I/Title. XV/Chapter 9 4 C https: //www. mass. gov/lists/standards-for-prescription-format-andsecurity Please direct any questions to: David E. Johnson, Director Drug Control Program Bureau of Health Professions Licensure dcp. dph@state. ma. us
- Slides: 11