Mass Transport NonIdeal Flow Reactors Advanced Transport Phenomena

- Slides: 44

Mass Transport: Non-Ideal Flow Reactors Advanced Transport Phenomena Module 6 - Lecture 28 Dr. R. Nagarajan Professor Dept of Chemical Engineering IIT Madras 1

MODELING OF NONIDEAL-FLOW REACTORS Ø Simplest approach: apply overall material/ energy/ momentum balances to the reactor Ø “black box’ approach, insufficient Ø Most rigorous: Divide into small subregions, approximate each region with PDEs Ø Impractical Ø Intermediate solution: model as discrete network of small number of interconnected ideal reactor types (SS PFR & WSR) 2

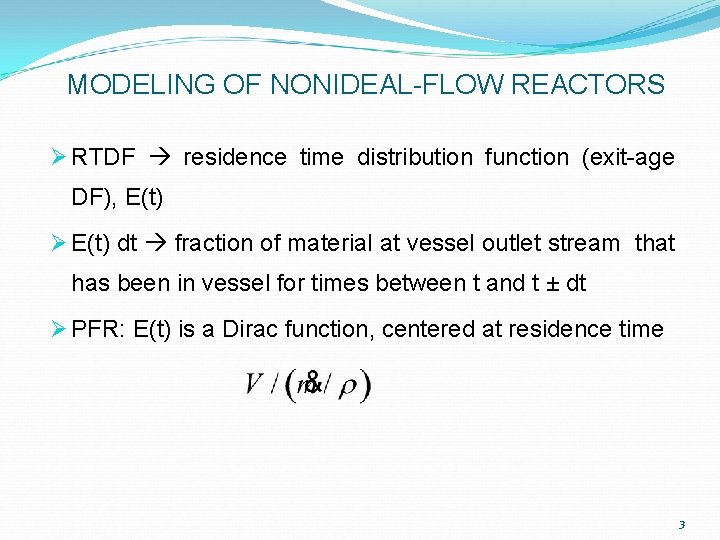
MODELING OF NONIDEAL-FLOW REACTORS Ø RTDF residence time distribution function (exit-age DF), E(t) Ø E(t) dt fraction of material at vessel outlet stream that has been in vessel for times between t and t ± dt Ø PFR: E(t) is a Dirac function, centered at residence time 3

MODELING OF NONIDEAL-FLOW REACTORS Ø V vessel volume Ø feed mass flow rate Ø e. g. , straight tube through which incompressible fluid flows with a uniform plug-flow velocity profile Ø Partial recycle can alter RTDF 4

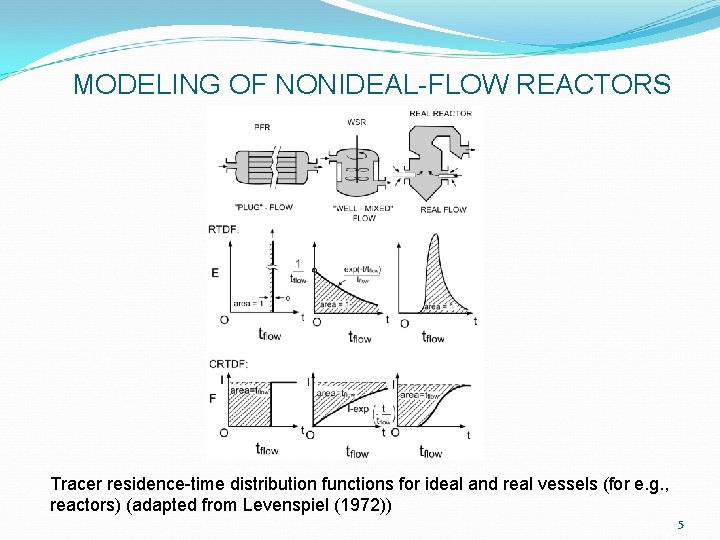
MODELING OF NONIDEAL-FLOW REACTORS Tracer residence-time distribution functions for ideal and real vessels (for e. g. , reactors) (adapted from Levenspiel (1972)) 5

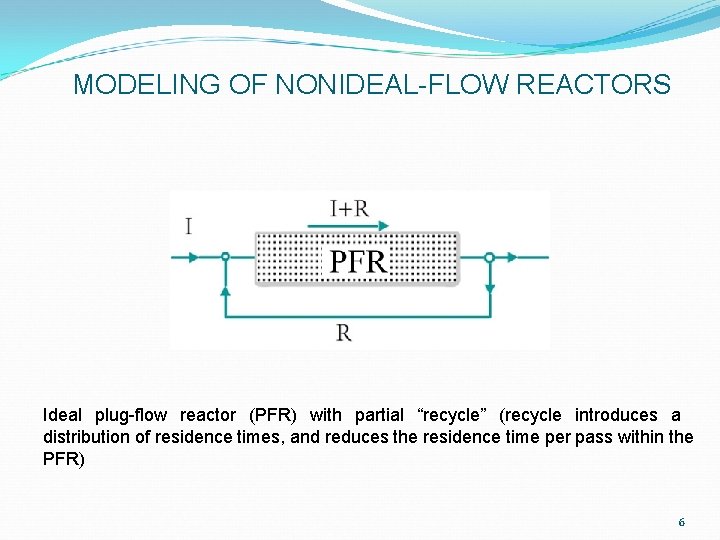
MODELING OF NONIDEAL-FLOW REACTORS Ideal plug-flow reactor (PFR) with partial “recycle” (recycle introduces a distribution of residence times, and reduces the residence time per pass within the PFR) 6

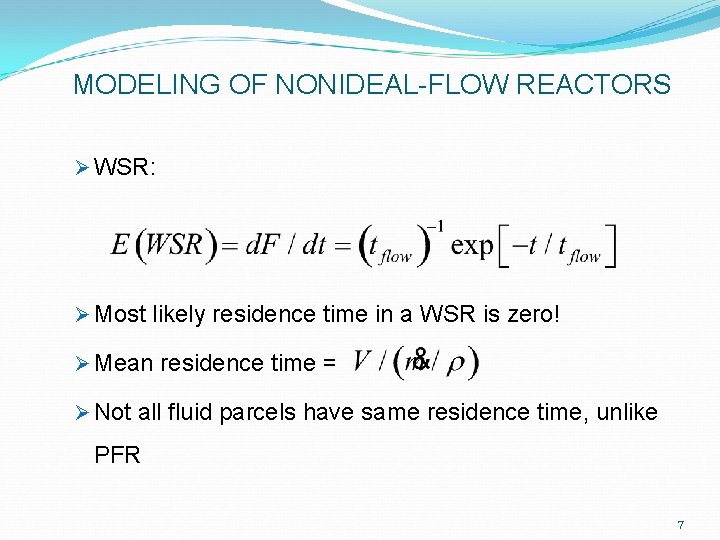
MODELING OF NONIDEAL-FLOW REACTORS Ø WSR: Ø Most likely residence time in a WSR is zero! Ø Mean residence time = Ø Not all fluid parcels have same residence time, unlike PFR 7

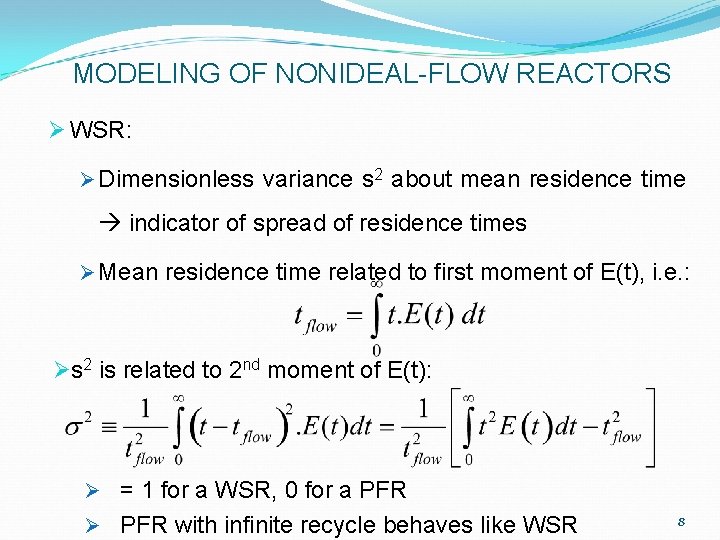
MODELING OF NONIDEAL-FLOW REACTORS Ø WSR: Ø Dimensionless variance s 2 about mean residence time indicator of spread of residence times Ø Mean residence time related to first moment of E(t), i. e. : Øs 2 is related to 2 nd moment of E(t): Ø = 1 for a WSR, 0 for a PFR Ø PFR with infinite recycle behaves like WSR 8

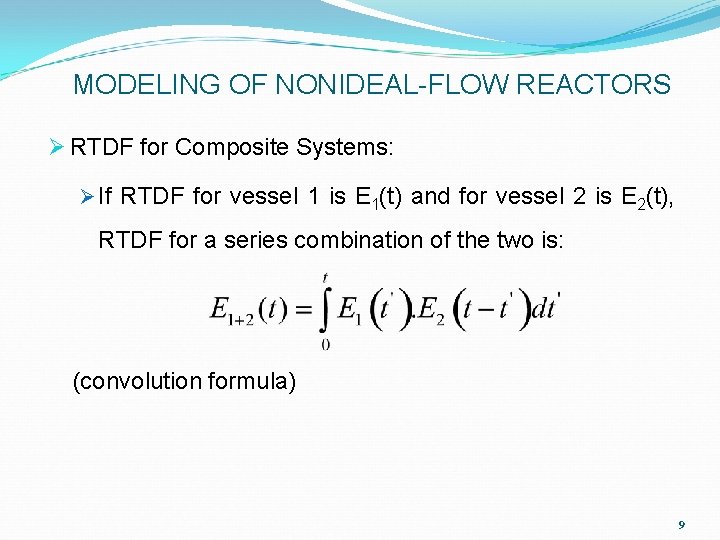
MODELING OF NONIDEAL-FLOW REACTORS Ø RTDF for Composite Systems: Ø If RTDF for vessel 1 is E 1(t) and for vessel 2 is E 2(t), RTDF for a series combination of the two is: (convolution formula) 9

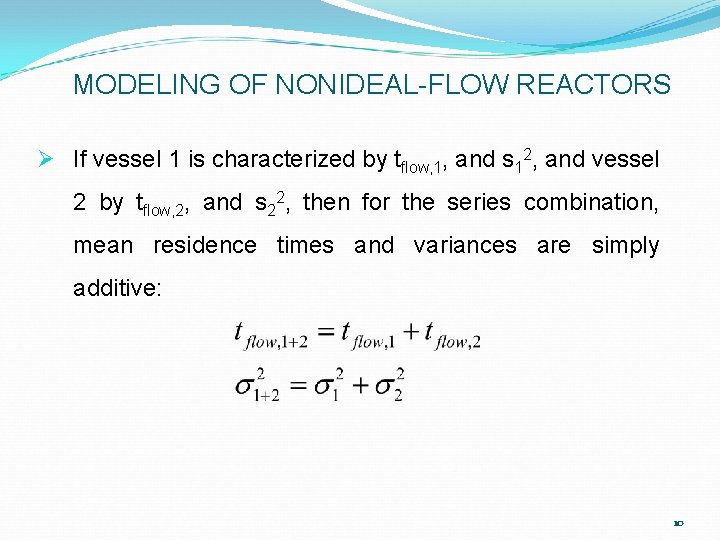
MODELING OF NONIDEAL-FLOW REACTORS Ø If vessel 1 is characterized by tflow, 1, and s 12, and vessel 2 by tflow, 2, and s 22, then for the series combination, mean residence times and variances are simply additive: 10

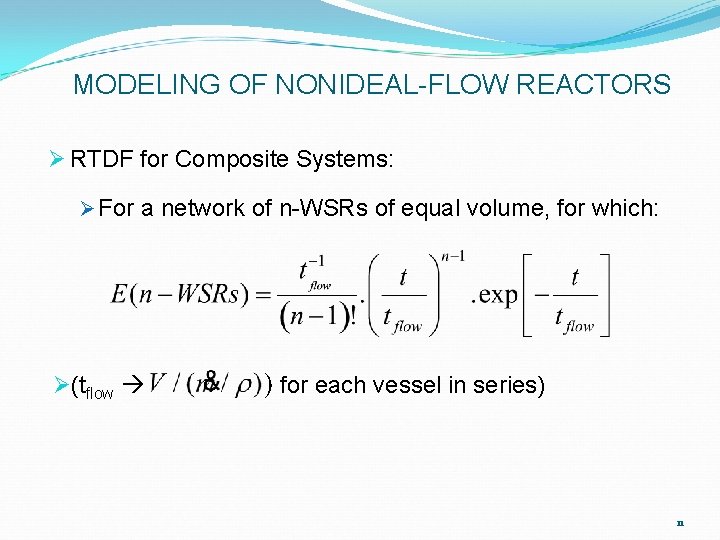
MODELING OF NONIDEAL-FLOW REACTORS Ø RTDF for Composite Systems: Ø For a network of n-WSRs of equal volume, for which: Ø(tflow ) for each vessel in series) 11

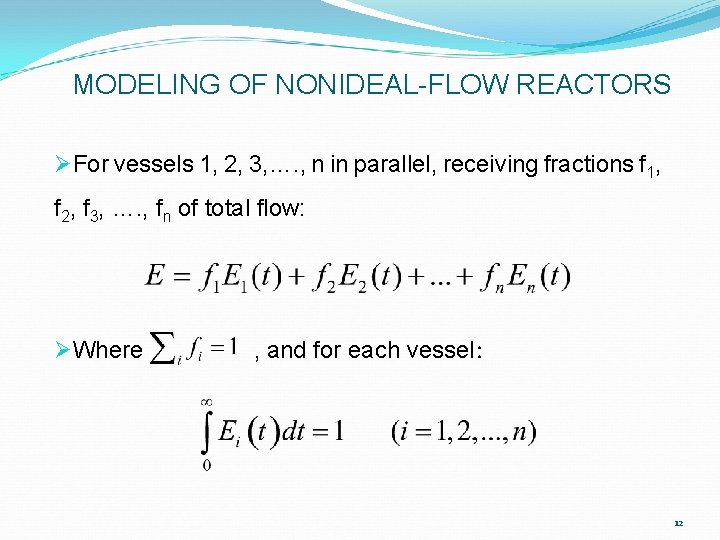
MODELING OF NONIDEAL-FLOW REACTORS ØFor vessels 1, 2, 3, …. , n in parallel, receiving fractions f 1, f 2, f 3, …. , fn of total flow: ØWhere , and for each vessel: 12

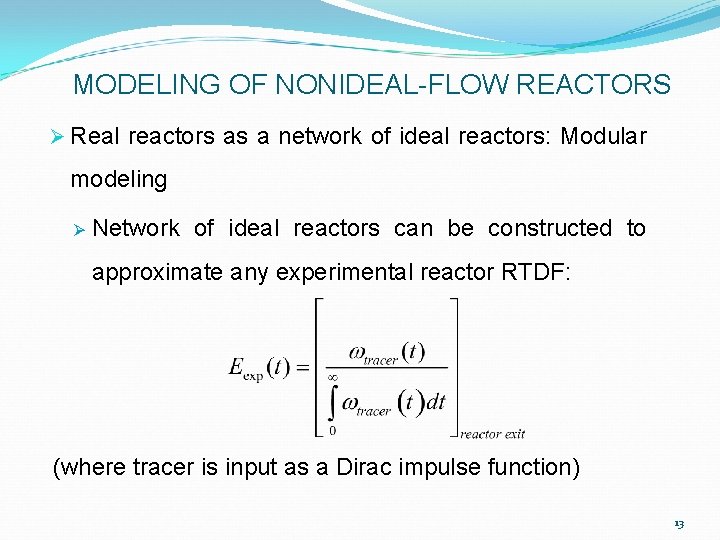
MODELING OF NONIDEAL-FLOW REACTORS Ø Real reactors as a network of ideal reactors: Modular modeling Ø Network of ideal reactors can be constructed to approximate any experimental reactor RTDF: (where tracer is input as a Dirac impulse function) 13

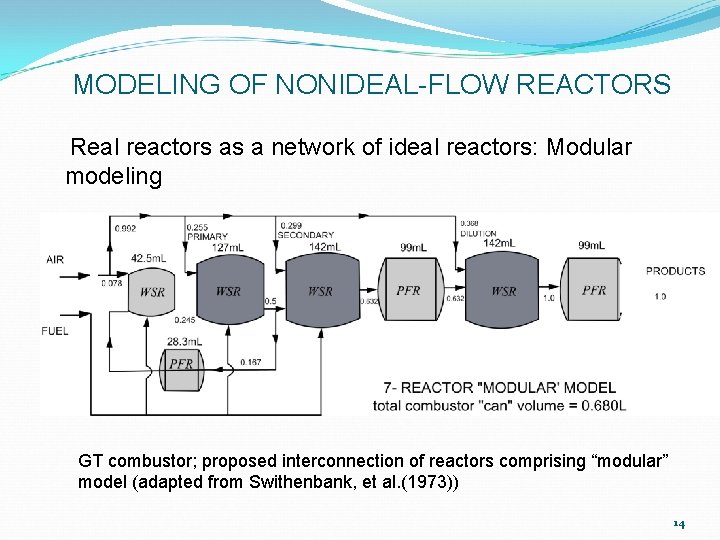
MODELING OF NONIDEAL-FLOW REACTORS Real reactors as a network of ideal reactors: Modular modeling GT combustor; proposed interconnection of reactors comprising “modular” model (adapted from Swithenbank, et al. (1973)) 14

MODELING OF NONIDEAL-FLOW REACTORS Ø Real reactors as a network of ideal reactors: Modular modeling Ø Info obtained from tracer diagnostics & from combustor geometry, cold-flow data, etc. Ø Important since RTD-data alone cannot discriminate between alternative networks with identical RTDmoments 15

MODELING OF NONIDEAL-FLOW REACTORS Ø Equivalent vessel network is nonunique Ø Each alternative may capture one aspect (e. g. , combustor efficiency) but not another (e. g. , domain of stable operation) 16

MODELING OF NONIDEAL-FLOW REACTORS Ø Real reactors as a network of ideal reactors: Modular modeling Ø Tracer methods can: Ø Guide development of “modular” models Ø Diagnose operating problems with existing chemical reactors or physical contactors Ø RTD data can show up dead-volumes, flowchanneling, bypassing (all cause inefficient operation) Ø Geometric or fluid-dynamic changes in design can correct these flaws Ø Perturbation in feed can be used as “tracer” 17

MODELING OF NONIDEAL-FLOW REACTORS Ø Real reactors as a network of ideal reactors: Modular modeling Ø RTD function, E(t), does concentration fluctuations not capture role of due to turbulence, incomplete mixing (at molecular level– “micromixing”) Ø When tracer concentration fluctuates at reactor exit, we only collect data on <E(t)> arithmetic average of N tracer shots, each yielding RTD Ej(t) (j = 1, 2, …. , N) 18

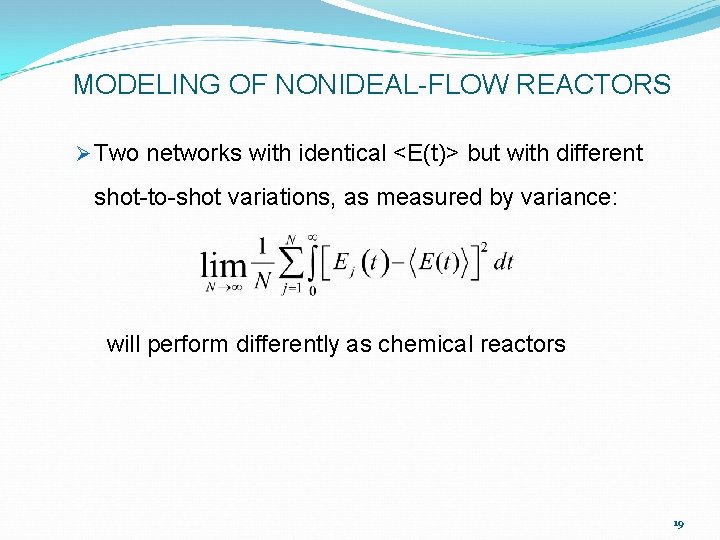
MODELING OF NONIDEAL-FLOW REACTORS Ø Two networks with identical <E(t)> but with different shot-to-shot variations, as measured by variance: will perform differently as chemical reactors 19

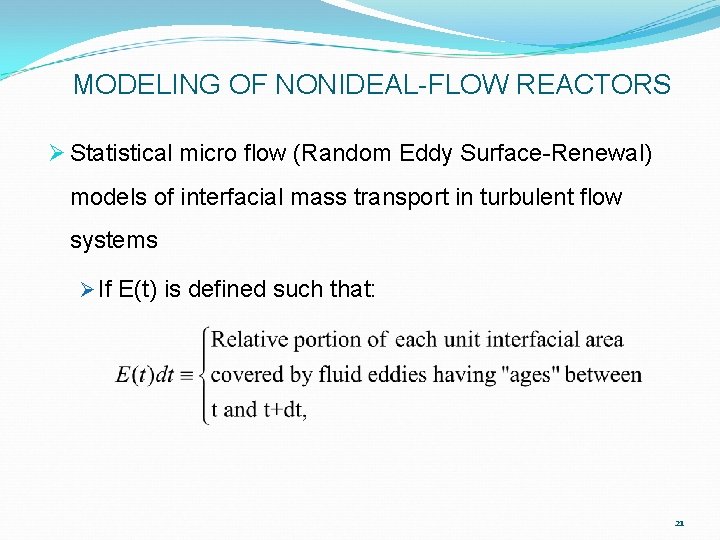
MODELING OF NONIDEAL-FLOW REACTORS Ø Statistical micro flow (Random Eddy Surface-Renewal) models of interfacial mass transport in turbulent flow systems Ø Mass/ energy transport visualized to occur during intervals of contact between turbulent eddies & surface Ø “stale” eddies replaced by fresh ones Ø Effective transport coefficient calculated by time- averaging RTDF-weighted instantaneous St(t) 20

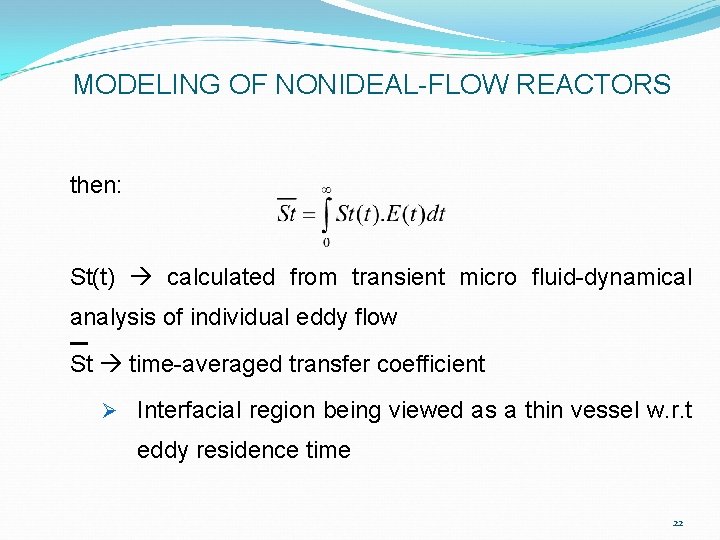
MODELING OF NONIDEAL-FLOW REACTORS Ø Statistical micro flow (Random Eddy Surface-Renewal) models of interfacial mass transport in turbulent flow systems Ø If E(t) is defined such that: 21

MODELING OF NONIDEAL-FLOW REACTORS then: St(t) calculated from transient micro fluid-dynamical analysis of individual eddy flow St time-averaged transfer coefficient Ø Interfacial region being viewed as a thin vessel w. r. t eddy residence time 22

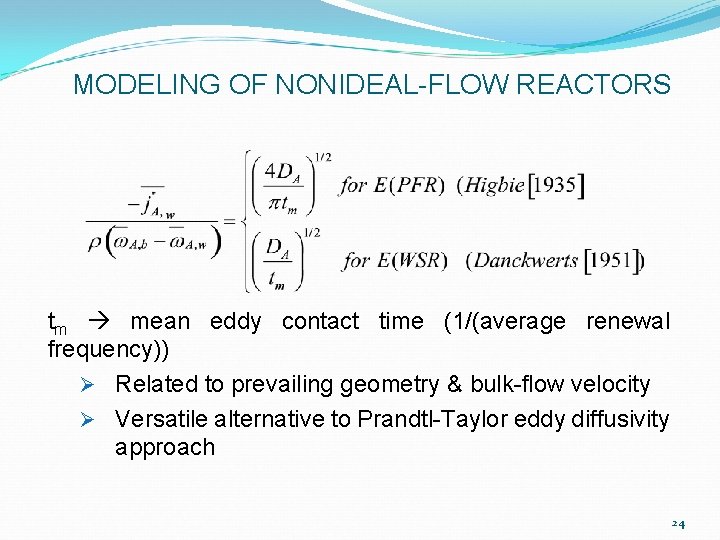
MODELING OF NONIDEAL-FLOW REACTORS Ø Statistical microflow (Random Eddy Surface-Renewal) models of interfacial mass transport in turbulent flow systems Ø Earliest & simplest model: each eddy considered to behave like a translating solid body Ø Large compared to transient diffusion BL (penetration) thickness Ø Dimensional time-averaged mass-transfer coefficient given by: 23

MODELING OF NONIDEAL-FLOW REACTORS tm mean eddy contact time (1/(average renewal frequency)) Ø Related to prevailing geometry & bulk-flow velocity Ø Versatile alternative to Prandtl-Taylor eddy diffusivity approach 24

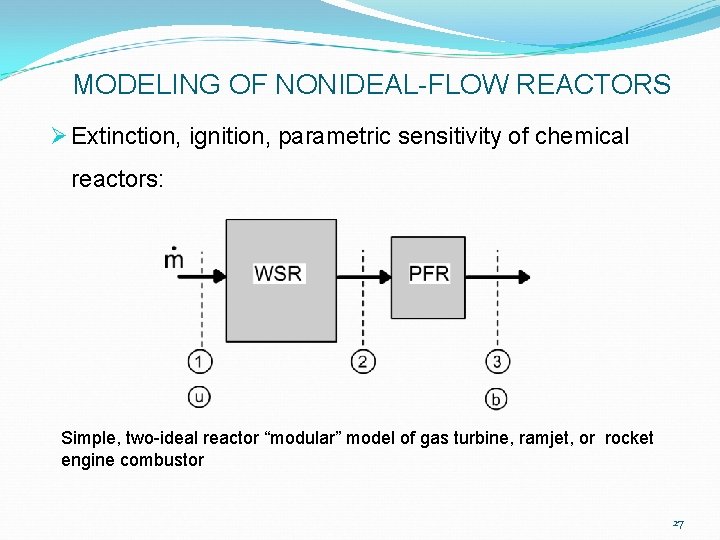
MODELING OF NONIDEAL-FLOW REACTORS Ø Extinction, ignition, parametric sensitivity of chemical reactors: Ø Simplest modular model for steady-flow behavior of combustors: WSR + PFR 25

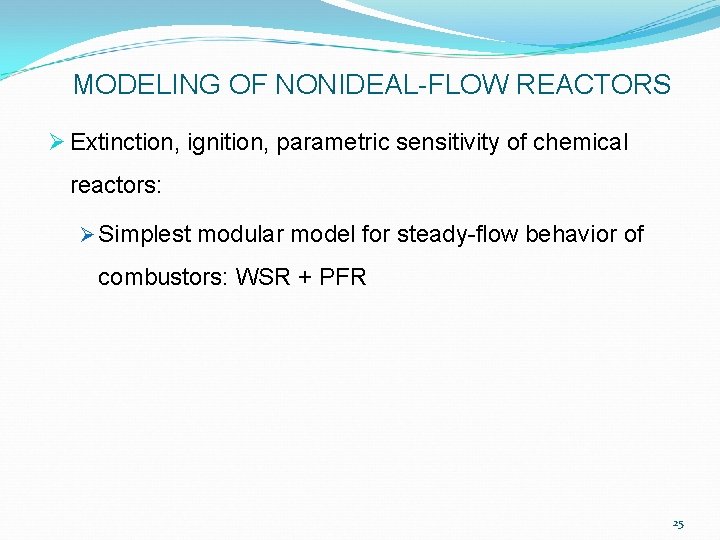
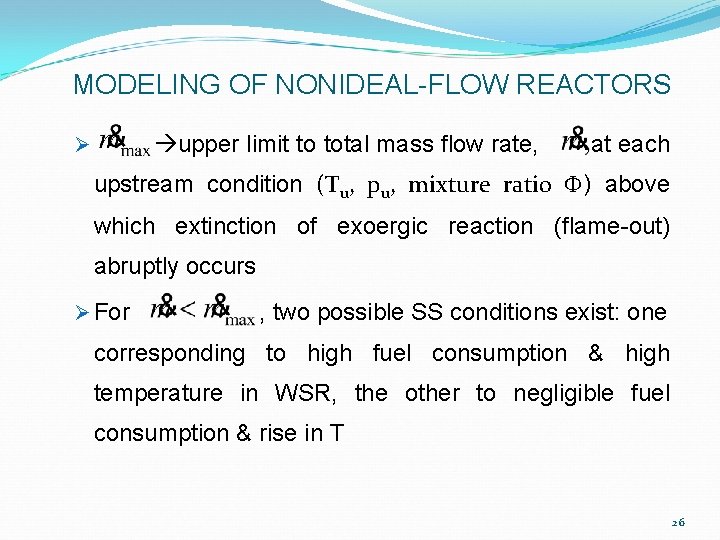
MODELING OF NONIDEAL-FLOW REACTORS upper limit to total mass flow rate, Ø at each upstream condition (Tu, pu, mixture ratio F) above which extinction of exoergic reaction (flame-out) abruptly occurs Ø For , two possible SS conditions exist: one corresponding to high fuel consumption & high temperature in WSR, the other to negligible fuel consumption & rise in T 26

MODELING OF NONIDEAL-FLOW REACTORS Ø Extinction, ignition, parametric sensitivity of chemical reactors: Simple, two-ideal reactor “modular” model of gas turbine, ramjet, or rocket engine combustor 27

MODELING OF NONIDEAL-FLOW REACTORS Ø Extinction, ignition, parametric sensitivity of chemical reactors: Ø Parametric sensitivity: change in reactor performance for a small change in input or operating parameter (e. g. , Tu) 28

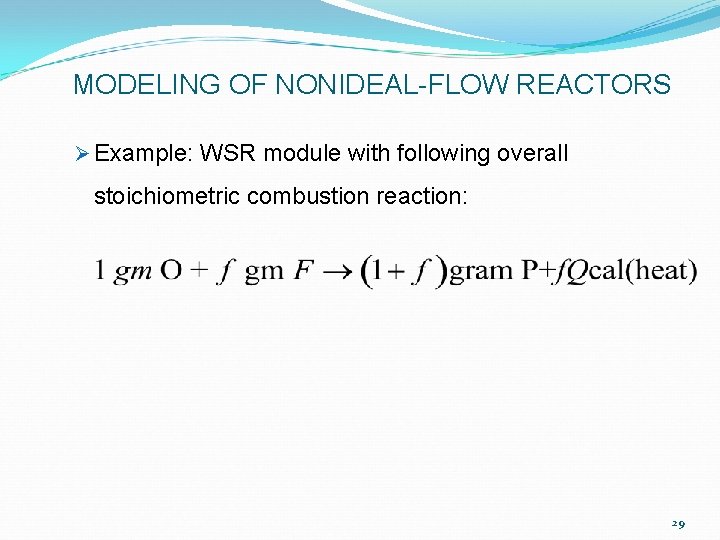
MODELING OF NONIDEAL-FLOW REACTORS Ø Example: WSR module with following overall stoichiometric combustion reaction: 29

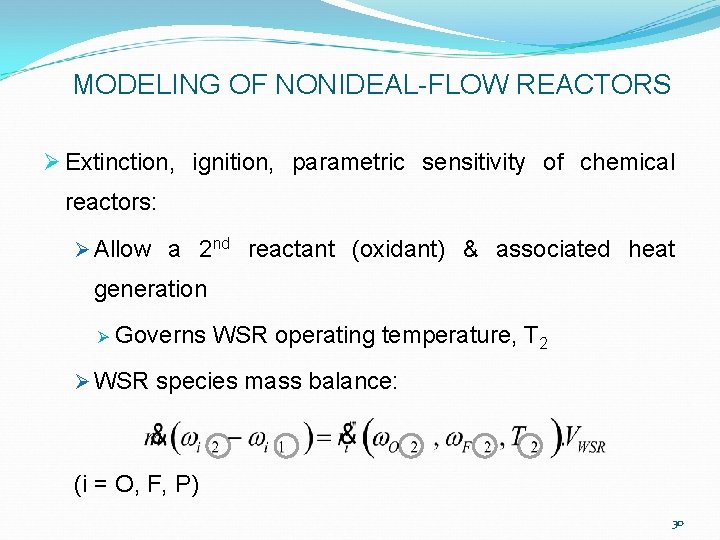
MODELING OF NONIDEAL-FLOW REACTORS Ø Extinction, ignition, parametric sensitivity of chemical reactors: Ø Allow a 2 nd reactant (oxidant) & associated heat generation Ø Governs WSR operating temperature, T 2 Ø WSR species mass balance: (i = O, F, P) 30

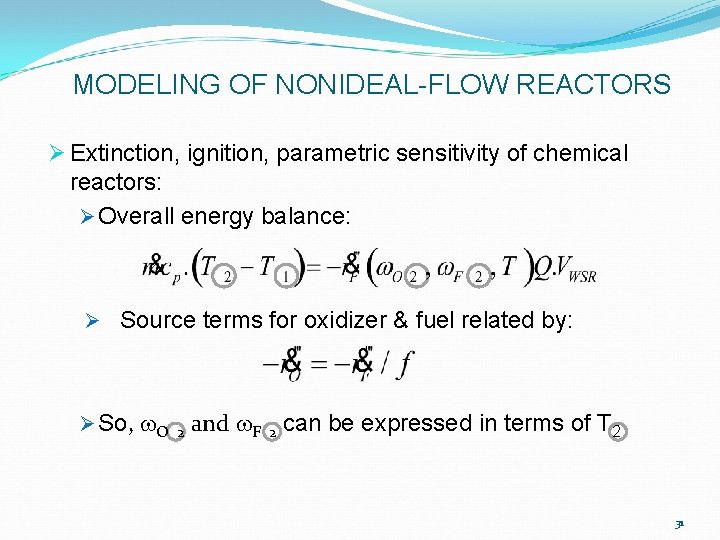
MODELING OF NONIDEAL-FLOW REACTORS Ø Extinction, ignition, parametric sensitivity of chemical reactors: Ø Overall energy balance: Ø Source terms for oxidizer & fuel related by: Ø So, w. O 2 and w. F 2 can be expressed in terms of T 2 31

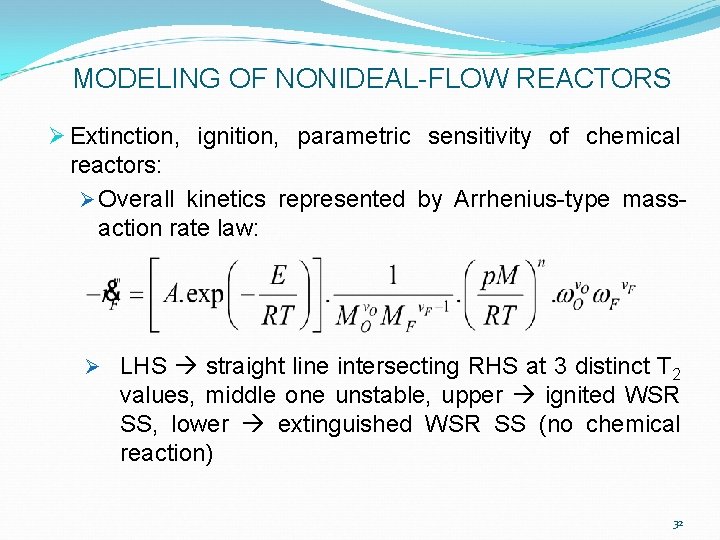
MODELING OF NONIDEAL-FLOW REACTORS Ø Extinction, ignition, parametric sensitivity of chemical reactors: Ø Overall kinetics represented by Arrhenius-type massaction rate law: Ø LHS straight line intersecting RHS at 3 distinct T 2 values, middle one unstable, upper ignited WSR SS, lower extinguished WSR SS (no chemical reaction) 32

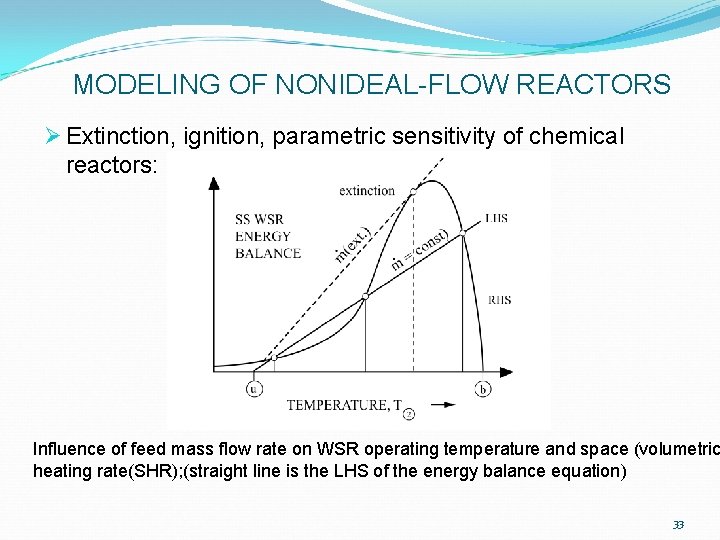
MODELING OF NONIDEAL-FLOW REACTORS Ø Extinction, ignition, parametric sensitivity of chemical reactors: Influence of feed mass flow rate on WSR operating temperature and space (volumetric heating rate(SHR); (straight line is the LHS of the energy balance equation) 33

MODELING OF NONIDEAL-FLOW REACTORS Ø Extinction, ignition, parametric sensitivity of chemical reactors: Ø Maximum volumetric rate of fuel consumption (hence, maximum chemical heating rate) occurs at WSR temperature: Ø Only slightly > “extinction” temperature (previous Figure) ØTb adiabatic, complete-combustion temperature Ø Typical E, n values listed in following Table 34

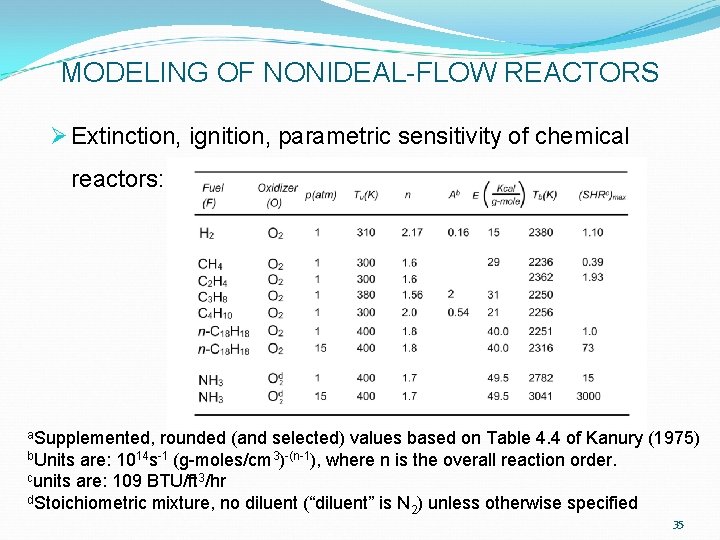
MODELING OF NONIDEAL-FLOW REACTORS Ø Extinction, ignition, parametric sensitivity of chemical reactors: a. Supplemented, rounded (and selected) values based on Table 4. 4 of Kanury (1975) b. Units are: 1014 s-1 (g-moles/cm 3)-(n-1), where n is the overall reaction order. cunits are: 109 BTU/ft 3/hr d. Stoichiometric mixture, no diluent (“diluent” is N ) unless otherwise specified 2 35

MODELING OF NONIDEAL-FLOW REACTORS Ø Extinction, ignition, parametric sensitivity of chemical reactors: Ø Black-box modular-models capture many important features of real reactors, useful for correlating performance data on full-scale & small-scale models Ø Predictive ability limited compared to more-detailed pseudo-continuum mathematical models Ø All have, as their basis, macroscopic conservation principles outlined earlier in this course. 36

PROBLEM 1 Ø The length requirement for a honeycomb-type automotive exhaust catalytic converter is set by the need to reduce the CO concentration in the exhaust to about 5% of the inlet concentration (i. e. , 95% conversion). Consider the basic conditions: Inlet gas temperature Inlet gas pressure Inlet gas composition (mole fraction) 700 K 1 atm y(N 2)=0. 93, y(CO)=0. 02, y(O 2)=0. 05 37

PROBLEM 1 Inlet gas velocity 103 cm/s Channel cross-section dimensions 1. 5 mm by 1. 5 mm (each channel) Assumed channel wall temperature 500 K Assume that the Pt-based catalyst used on the walls of each channel is active enough to cause the surfacecatalyzed CO oxidation reaction to be diffusion-controlled, that is, the steady-state value of the CO-mass fraction established at (1 mean-free-path away from) 38

PROBLEM 1 the wall, w. CO, w , is negligible compared to w. CO, b(z) within each channel. Also assume that the gas-phase kinetics of CO oxidation under these conditions preclude appreciable (uncatalyzed) homogeneous CO-assumption in the available residence times. Answer the following questions: a. By what mechanism is CO(g) mass transported to the channel wall, where chemical consumption (to produce CO 2) occurs? What is the relevant transport coefficient 39

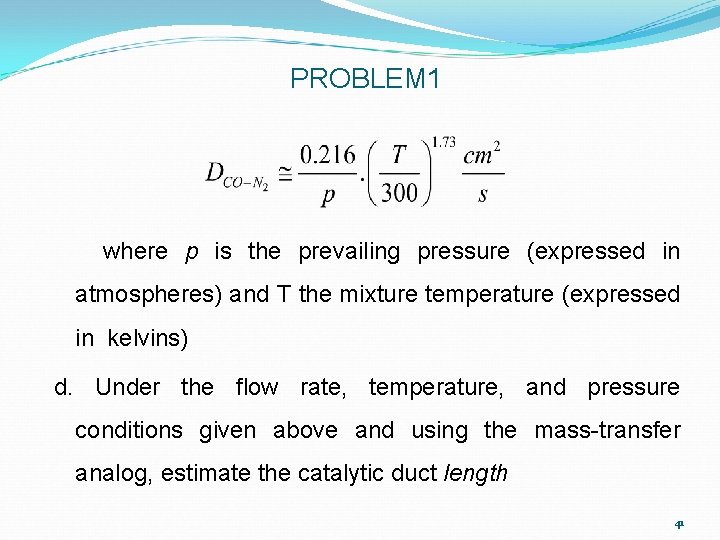
PROBLEM 1 and to what energy-transfer process and transport properly coefficient is this “analogous”? b. Are the mass-heat transfer analogy conditions (MAC, HAC) discussed in this module approximately met in this application? What is the inlet mass fraction of CO gas? c. Estimate the Schmidt number mix for CO Fick diffusion through the prevailing combustion gas mixture, using the experimental observation that 40

PROBLEM 1 where p is the prevailing pressure (expressed in atmospheres) and T the mixture temperature (expressed in kelvins) d. Under the flow rate, temperature, and pressure conditions given above and using the mass-transfer analog, estimate the catalytic duct length 41

PROBLEM 1 required to consume 95% of the inlet CO concentration, and the mixing cup (bulk) stream temperature at this length. e. List and defend the principal assumptions made in arriving at the length estimate (of Part (d)) f. If the catalyst were “poisoned” (e. g. , by lead compounds), what could happen to the CO exit concentration? Which of the assumptions used in predicting the required converter length (Part (d)) would be violated? 42

PROBLEM 1 g. If the heat of combustion of CO(g) is about 67. 8 kcal/gmole CO consumed, calculate how much must be removed to maintain the channel-wall temperature constant at 500 K? h. Automatic operating conditions are never strictly steady, so that in practice the mass-flow rate, temperature, and gas composition entering the catalytic afterburner will be time-dependent. Under what circumstances (be 43

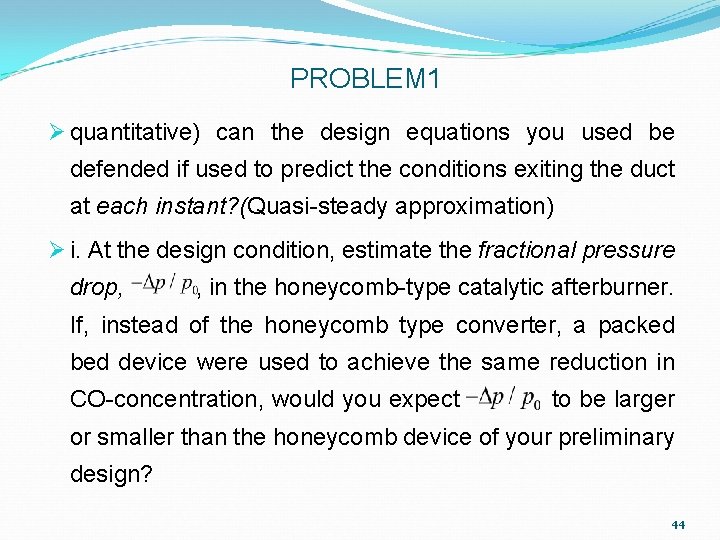
PROBLEM 1 Ø quantitative) can the design equations you used be defended if used to predict the conditions exiting the duct at each instant? (Quasi-steady approximation) Ø i. At the design condition, estimate the fractional pressure drop, , in the honeycomb-type catalytic afterburner. If, instead of the honeycomb type converter, a packed bed device were used to achieve the same reduction in CO-concentration, would you expect to be larger or smaller than the honeycomb device of your preliminary design? 44