Mass Spectroscopy Mass Spectrometry Most useful tool for

















- Slides: 29


Mass Spectroscopy

Mass Spectrometry ä Most useful tool for molecular structure determination if you can get it into gas phase ä Molecular weight of parent and fragments that can give hints as to the structure of the molecule

Early Use of Mass Spectroscopy ä Quantitative methods for determination of the components in complex hydrocarbon mixtures ä Later used for the identification and structural analysis of complex compounds

Principles of measurements ä As an identification method: ä When a given molecular species is impacted with an electron beam, a family of positive particles are produced ä The mass distribution of the particles are characteristic of the parent species

How does it work? ä Sample is volatized and allowed to leak slowly into an ion chamber ä Molecules of sample are ionized to mostly positive ions by electron beam ä Positive ions are separated from negative ions

How does it work? ä Positive ions are accelerated into separation chamber ä The fast moving particles are subjected to a strong magnetic field in which they travel in a curved path ä The radius depends upon their velocity and mass as well as the field strength

How does it work? ä ions pass through an exit slit and fall upon a collector electrode ä the ion current that results is amplified and recorded as a function of field strength or accelerating potential

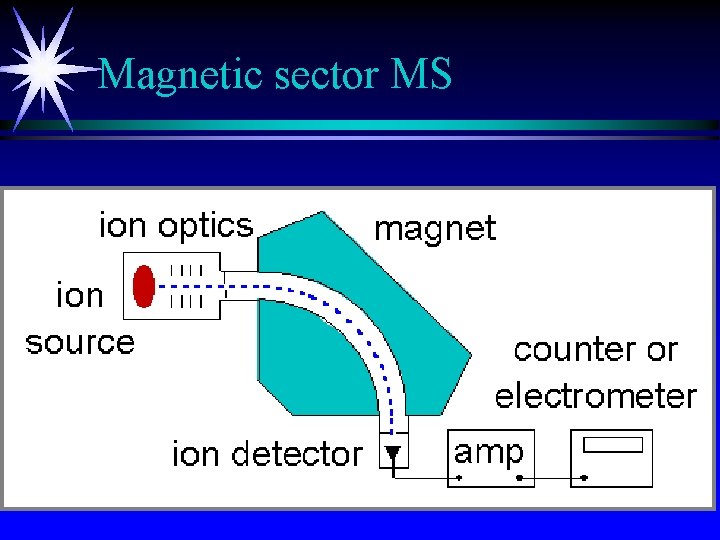
Magnetic sector MS

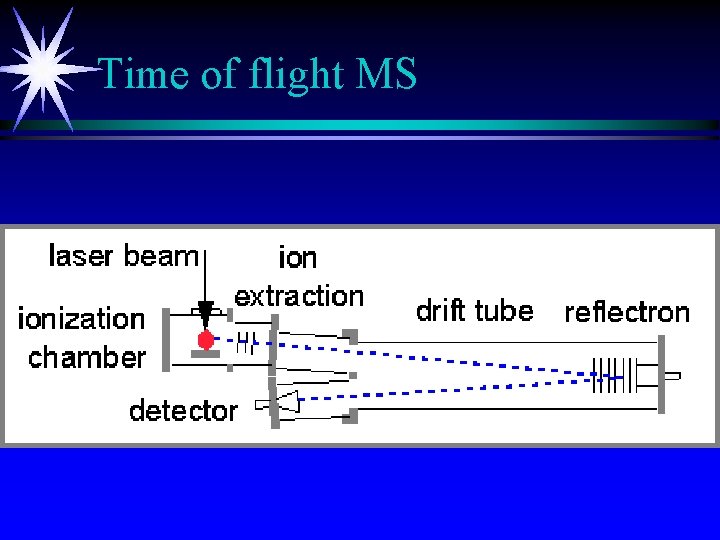
Time of flight MS


Portable TOF/MS

ICP/MS

Quadrupoles

Liquid Chrom GC Quadrupole MS

MS Instrumentation - Sample introduction ä Need high vacuum - 10 -6 10 torr( torr=1 mm Hg) ä Oil diffusion pumps, turbomolecular pump, diffusion pump ä Gas injection through pinhole ä Liquids that vaporize into vacuum ä Direct ionization of solids

Sample ionization ä General ionization that causes fragmentation (electron impact) ä Gentle ionization that favors intensity of molecular ion (chemical ionization with gaseous ions) ä Gentle ionization for molecules that are not in the gas phase (eg biological molecules) (Fast Atom Bombardment)

Ionization of inorganic solids ä Spark source (like atomic emission) ä ICP/MS ä Glow discharges (hollow cathode lamps) ä Laser spallation

Mass analyzer - separation based on mass to charge ratio ä Magnetic sector (single focus) ä Double focus (electrostatic field to select energy and magnetic field for m/e) ä Quadrupole (1 to 3) ä Time of flight (pulsed ioization) ä Fourier transform (ion cyclotron resonance)

Magnetic sector MS ä large ä slow scans (change magnetic field with electromagnet) ä finite spread of energies from source limit resolution ä R= m/Dm : capable of R= 600 to 2000 m/ which means looking at m/e of 600 with unit mass resolution

Double focus MS ä Very large with electrostatic analyzer to eliminate differences in KE of ions ä R= 40, 000 is possible which means for m/e of 100 the mass differences of 0. 0025 can be measured ä Isotopic differences: C 3 H 7 = 43. 0892 ä C 2 H 3 O= 43. 0456 ä C 2 H 5 N= 43. 0688

Quadrupole MS ä ä ä Electrical fields applied pairwise DC voltage and RF oscillating voltage changed so only one value of m/e moves in a stable path through the field Scans over 1000 mass units/sec R= 10, 000 possible R= 500 typical


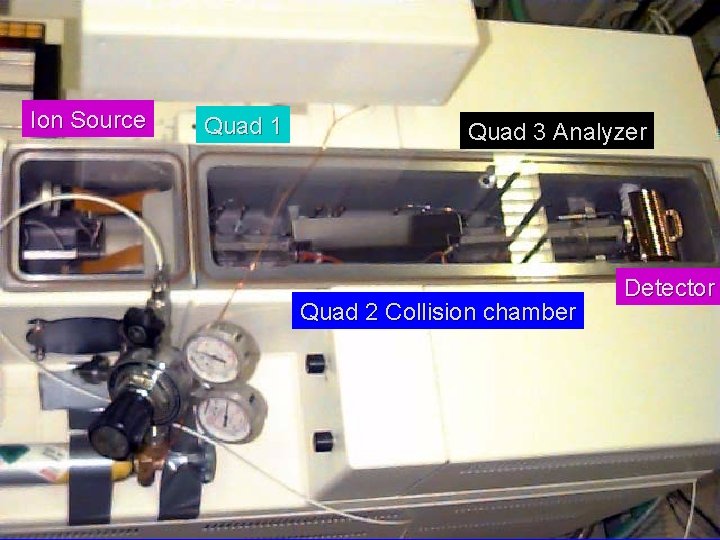
Ion Source Quad 1 Quad 3 Analyzer Quad 2 Collision chamber Detector

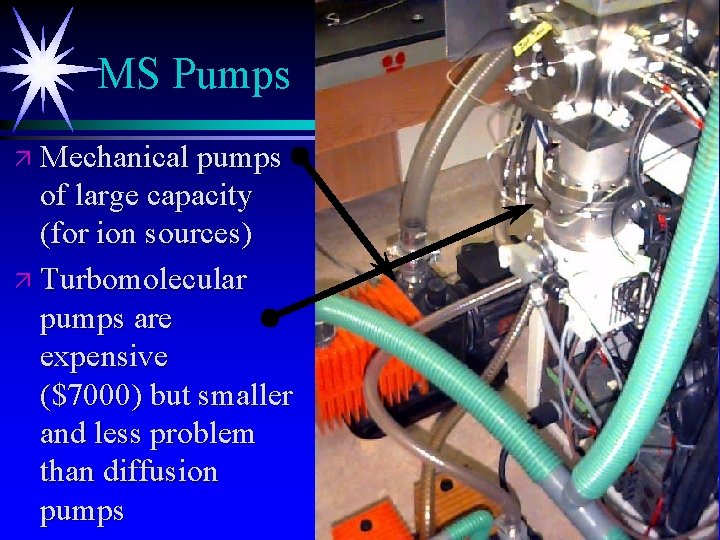
MS Pumps ä Mechanical pumps of large capacity (for ion sources) ä Turbomolecular pumps are expensive ($7000) but smaller and less problem than diffusion pumps

Ion Trap MS (like ion cyclotron)

Time of Flight MS ä Fast scans of pulses of ions ä e. V=1/2 mv 2 How long does it take for ions to travel 1 meter. ä R=400 at best ä Can store up to 20, 000 spectra/sec.

FT-MS or ICR-MS ä Simultaneous measurement, multiplex, mass accuracy, high throughput ä R= 800, 000 possible but expensive as you need superconducting magnet ä Different ions rotate at different velocities so we measure the frequencies ä m/e=H/2 p m/e=H/2 f

Triple Quadropole (MS-MS) ä Select an ion, fragment it, and then analyze the fragments Source Select ion Collision Chamber Analyze Detector