Mass Spectrometry Mass Spec Spectroscopic Methods for Structure





- Slides: 30

Mass Spectrometry (Mass Spec. )

Spectroscopic Methods for Structure Determination Ultraviolet-Visible (UV/Vis) spectroscopy: determination of solutions of transition metal ions and highly conjugated organic compounds Infrared (IR) spectroscopy: Functional groups Mass spectrometry (MS): Molecular mass and formula and structure information Nuclear magnetic resonance (NMR) spectroscopy: Map of carbon-hydrogen framework

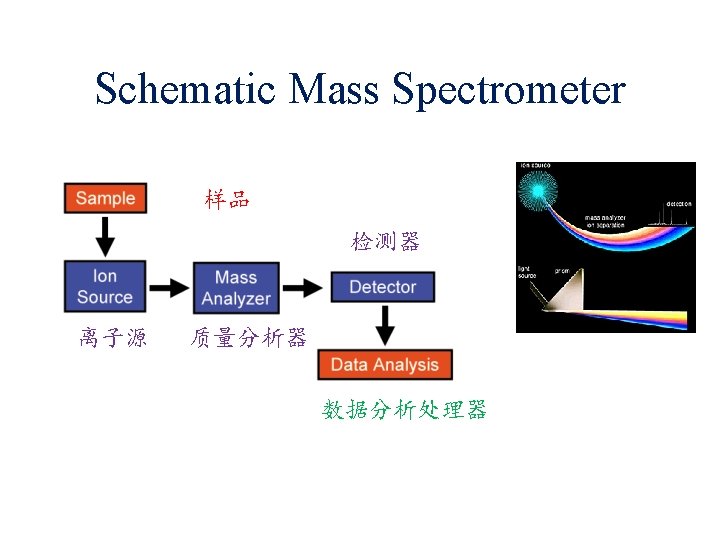
Definition of Mass Spectrometry Mass spectrometry (MS) : An analytical technique by using mass spectrometry for the determination of the composition of a sample or molecule and elucidation of the chemical structures of molecules, such as peptides and other chemical compounds.

By James Crawford How do two people with different languages communicate with each other? Then, how can I catch up, Ms. ?

What information can be determined? • Molecular weight • Molecular formula (HRMS) • Structure (from fragmentation fingerprint) • Isotopic incorporation / distribution • Protein sequence (MS-MS)

Fragmentation Patterns The impact of the stream of high energy • electrons often breaks the molecule into fragments, commonly a cation and a radical. Bonds break to give the most stable cation. – Stability of the radical is less important. –

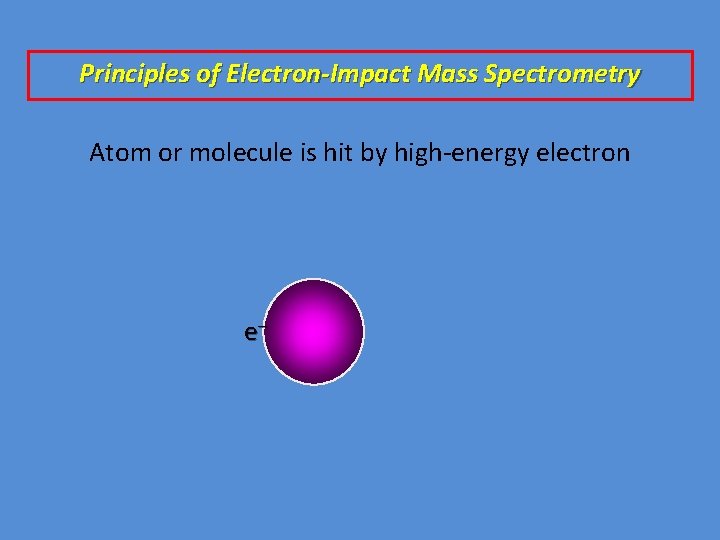
Principles of Electron-Impact Mass Spectrometry Atom or molecule is hit by high-energy electron e–

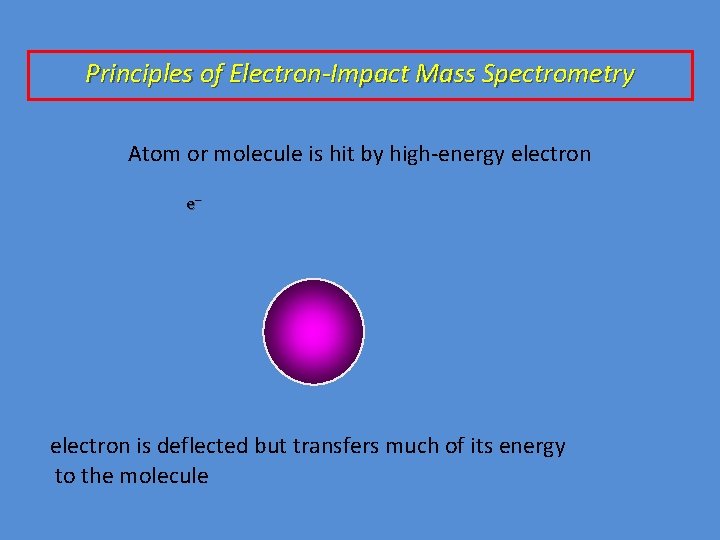
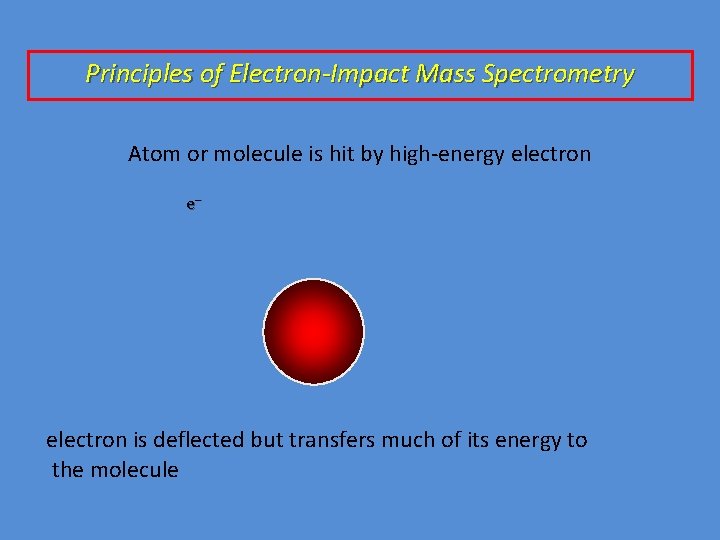
Principles of Electron-Impact Mass Spectrometry Atom or molecule is hit by high-energy electron e– electron is deflected but transfers much of its energy to the molecule

Principles of Electron-Impact Mass Spectrometry Atom or molecule is hit by high-energy electron e– electron is deflected but transfers much of its energy to the molecule

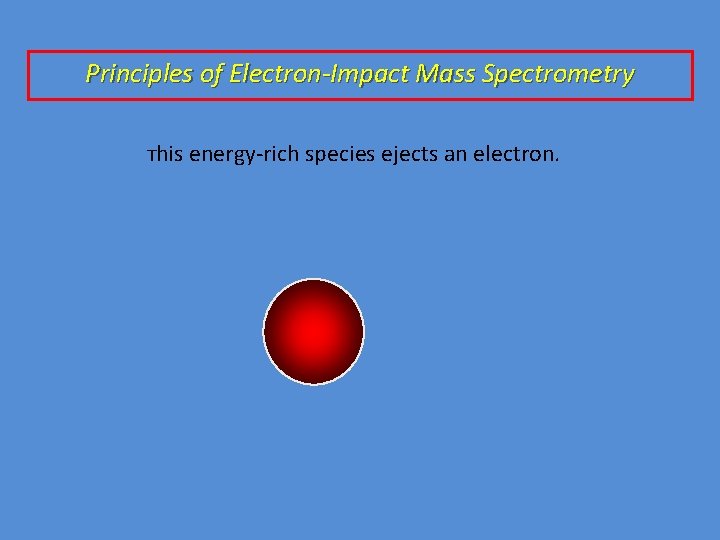
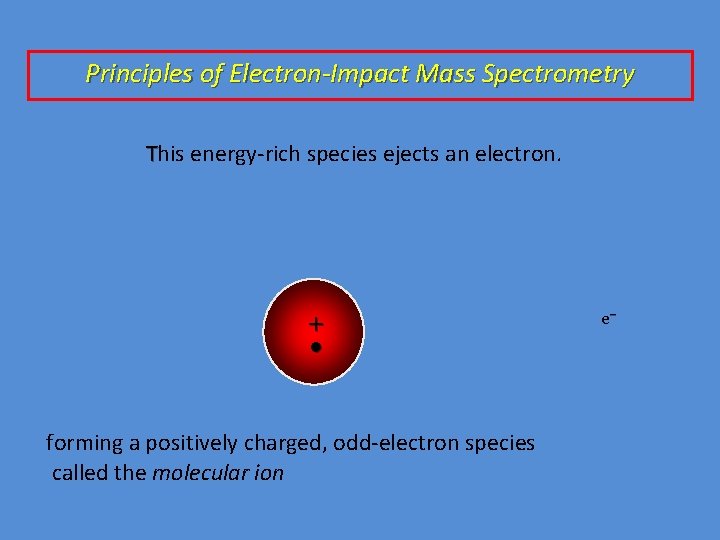
Principles of Electron-Impact Mass Spectrometry This energy-rich species ejects an electron.

Principles of Electron-Impact Mass Spectrometry This energy-rich species ejects an electron. + • forming a positively charged, odd-electron species called the molecular ion e–

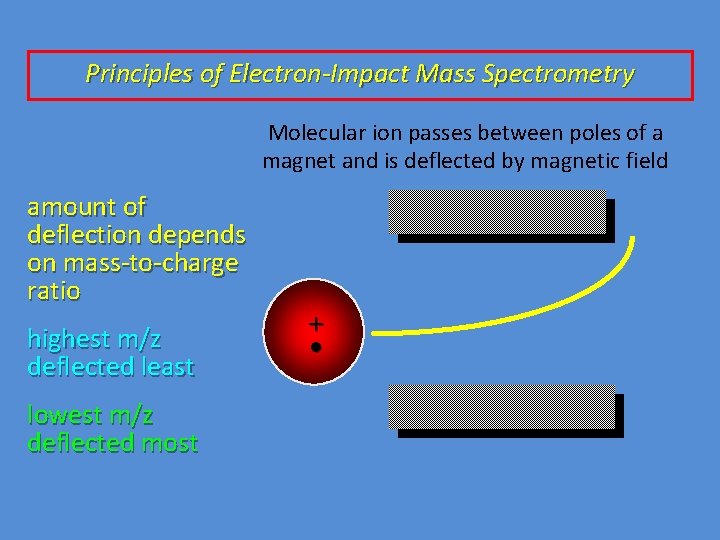
Principles of Electron-Impact Mass Spectrometry Molecular ion passes between poles of a magnet and is deflected by magnetic field amount of deflection depends on mass-to-charge ratio highest m/z deflected least lowest m/z deflected most + •

Principles of Electron-Impact Mass Spectrometry If the only ion that is present is the molecular ion, mass spectrometry provides a way to measure the molecular weight of a compound and is often used for this purpose. However, the molecular ion often fragments to a mixture of species of lower m/z.

Principles of Electron-Impact Mass Spectrometry The molecular ion dissociates to a cation and a radical. + •

Principles of Electron-Impact Mass Spectrometry The molecular ion dissociates to a cation and a radical. + • Usually several fragmentation pathways are available and a mixture of ions is produced.

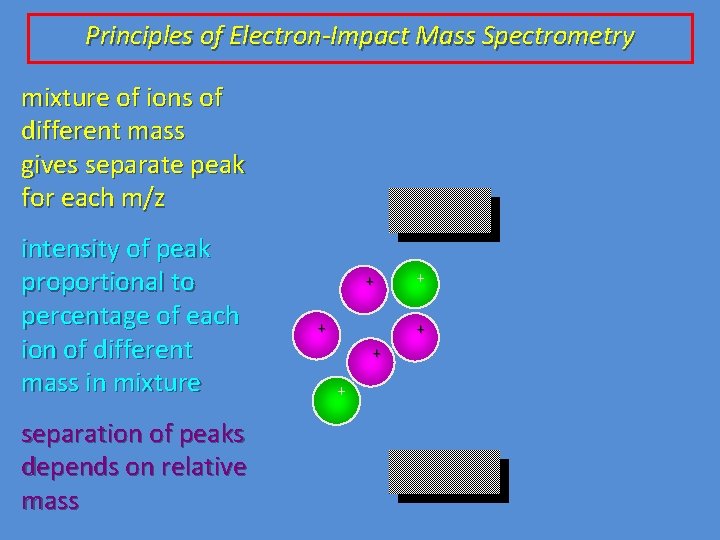
Principles of Electron-Impact Mass Spectrometry mixture of ions of different mass gives separate peak for each m/z intensity of peak proportional to percentage of each ion of different mass in mixture separation of peaks depends on relative mass + + +

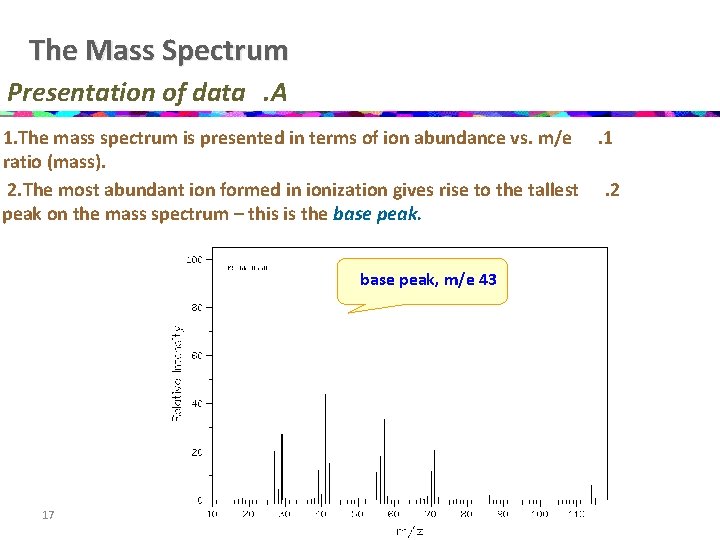
The Mass Spectrum Presentation of data. A 1. The mass spectrum is presented in terms of ion abundance vs. m/e. 1 ratio (mass). 2. The most abundant ion formed in ionization gives rise to the tallest. 2 peak on the mass spectrum – this is the base peak, m/e 43 17

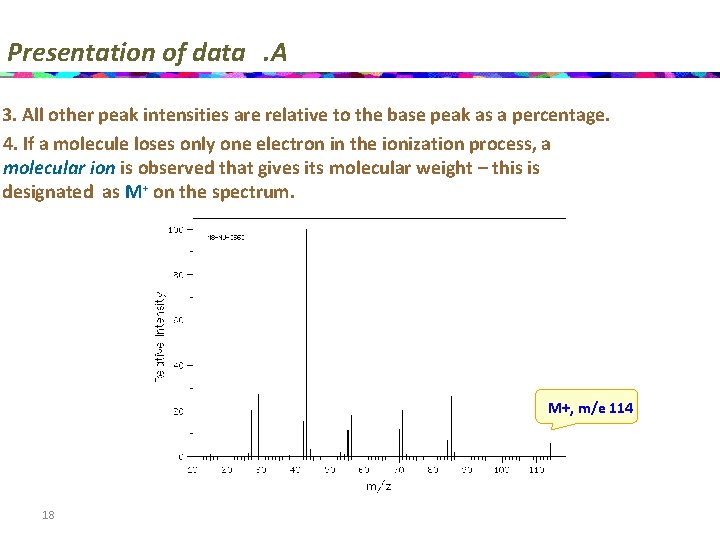
Presentation of data. A 3. All other peak intensities are relative to the base peak as a percentage. 4. If a molecule loses only one electron in the ionization process, a molecular ion is observed that gives its molecular weight – this is designated as M+ on the spectrum. M+, m/e 114 18

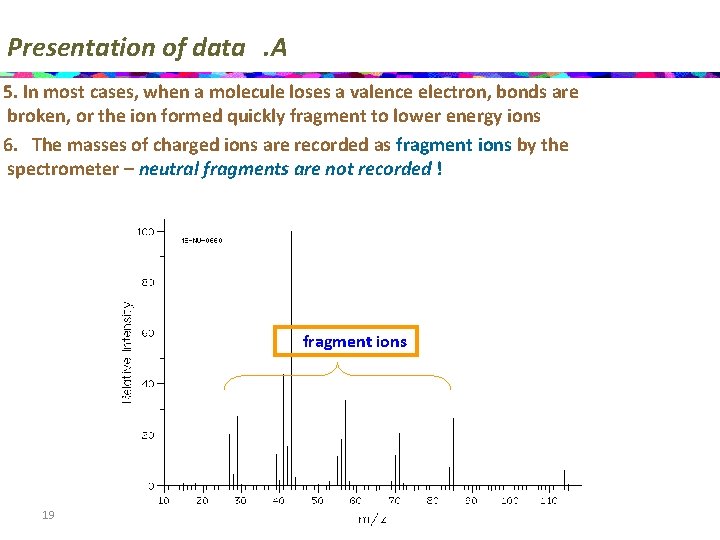
Presentation of data. A 5. In most cases, when a molecule loses a valence electron, bonds are broken, or the ion formed quickly fragment to lower energy ions 6. The masses of charged ions are recorded as fragment ions by the spectrometer – neutral fragments are not recorded ! fragment ions 19

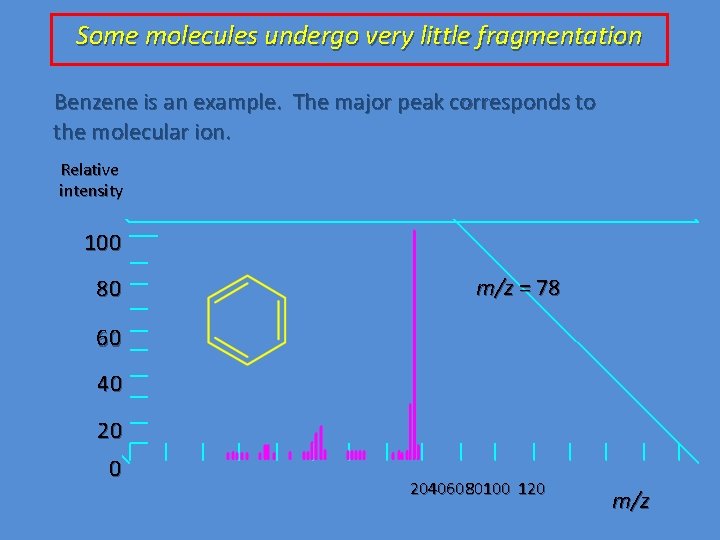
Some molecules undergo very little fragmentation Benzene is an example. The major peak corresponds to the molecular ion. Relative intensity 100 80 m/z = 78 60 40 20406080100 120 m/z

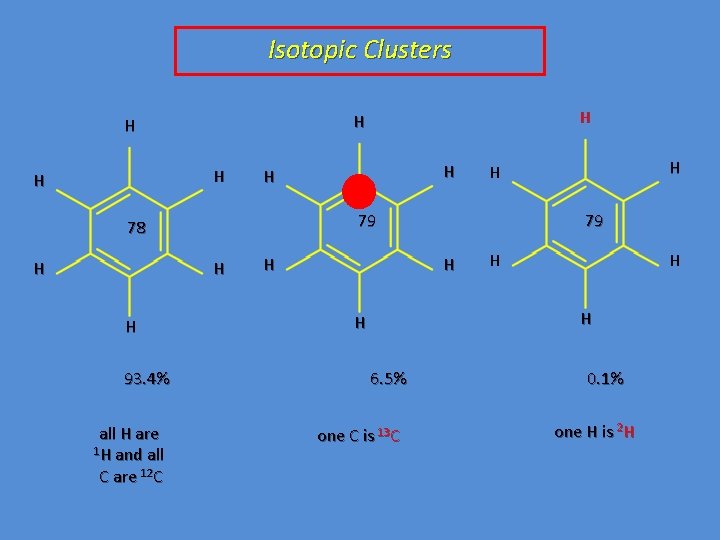
Isotopic Clusters H H 93. 4% all H are 1 H and all C are 12 C H H H 79 H H 78 H H 79 H H H 6. 5% one C is 13 C 0. 1% one H is 2 H

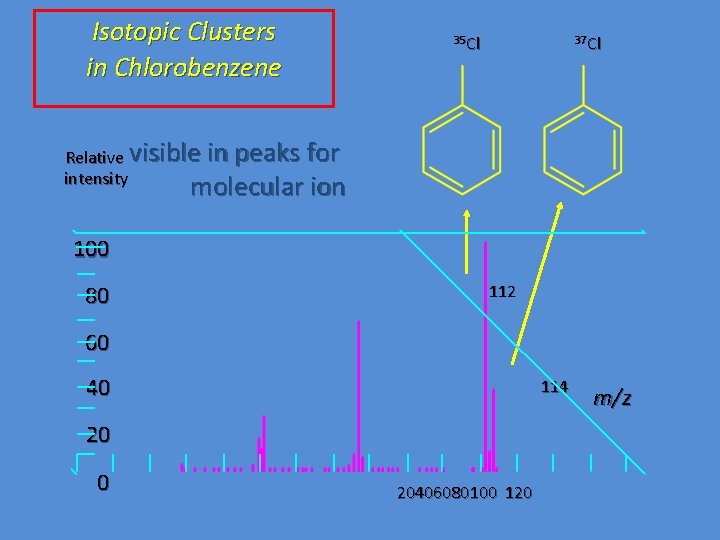
Isotopic Clusters in Chlorobenzene 35 Cl 37 Cl Relative visible in peaks for intensity molecular ion 100 80 112 60 40 114 20 0 20406080100 120 m/z

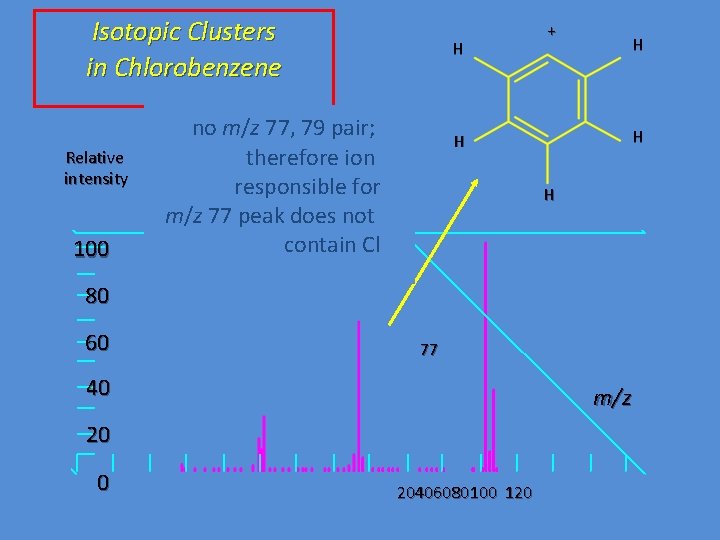
Isotopic Clusters in Chlorobenzene Relative intensity 100 H no m/z 77, 79 pair; therefore ion responsible for m/z 77 peak does not contain Cl + H H 80 60 77 40 m/z 20 0 20406080100 120



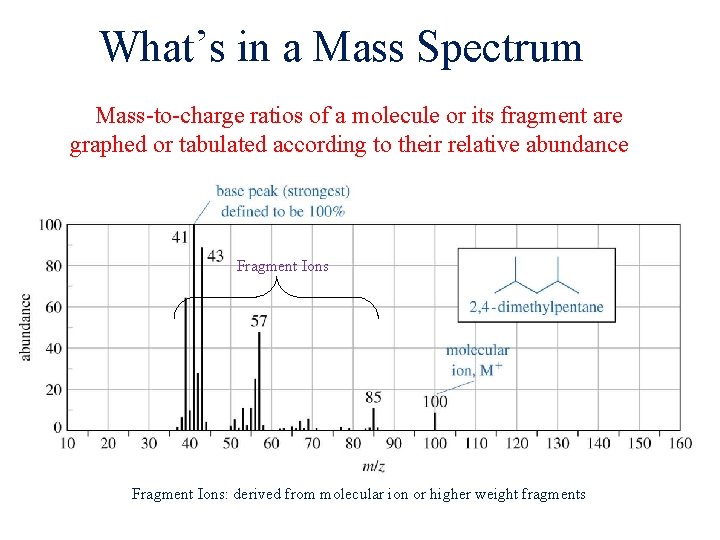
What’s in a Mass Spectrum Mass-to-charge ratios of a molecule or its fragment are graphed or tabulated according to their relative abundance Fragment Ions: derived from molecular ion or higher weight fragments

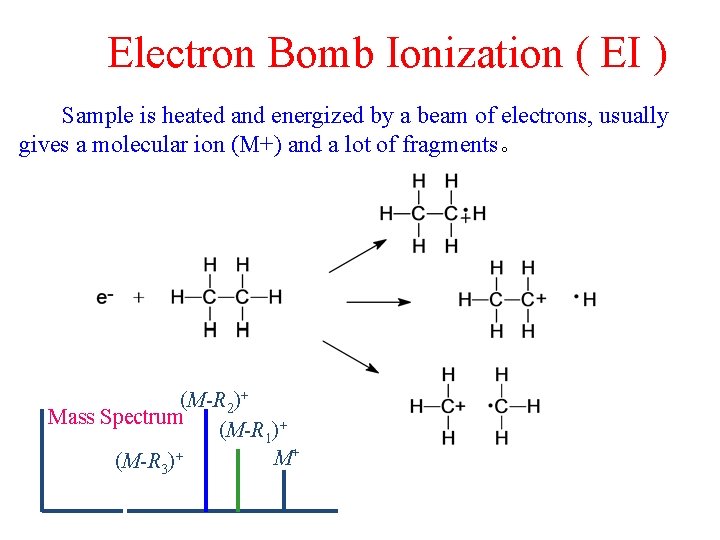
Electron Bomb Ionization ( EI ) Sample is heated and energized by a beam of electrons, usually gives a molecular ion (M+) and a lot of fragments。 (M-R 2)+ Mass Spectrum (M-R 1)+ M+ (M-R 3)+

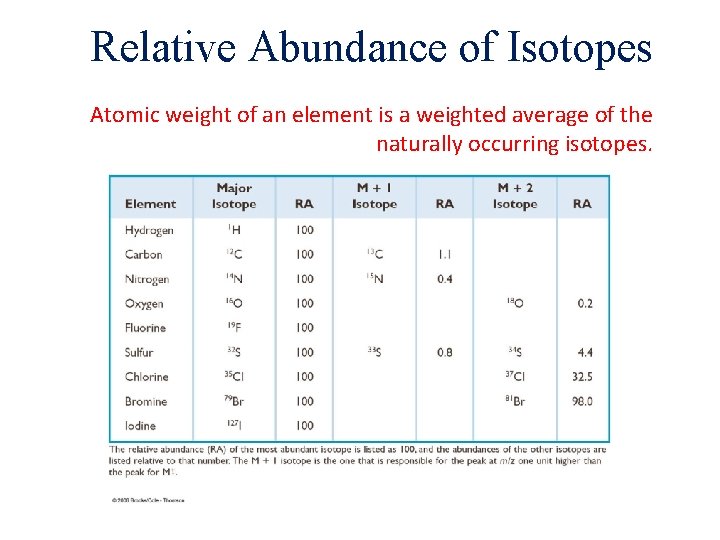
Relative Abundance of Isotopes Atomic weight of an element is a weighted average of the naturally occurring isotopes.

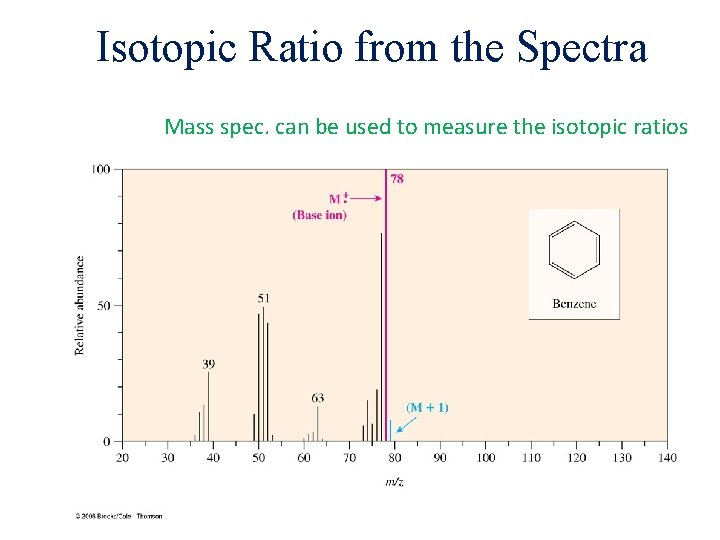
Isotopic Ratio from the Spectra Mass spec. can be used to measure the isotopic ratios

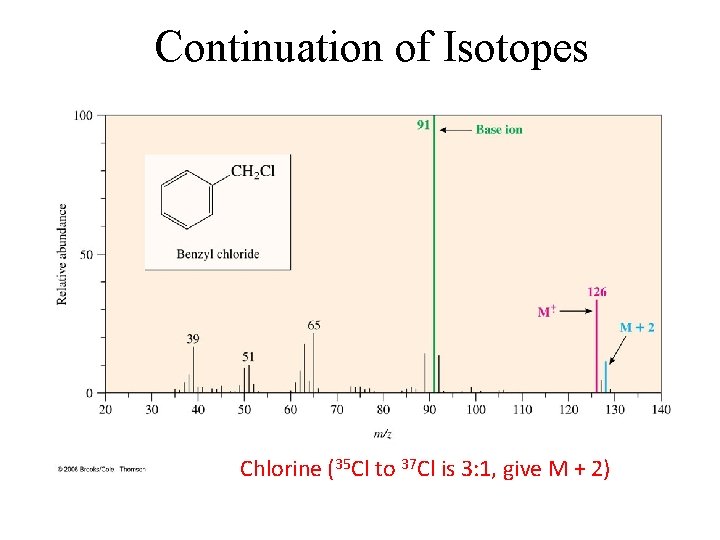
Continuation of Isotopes Chlorine (35 Cl to 37 Cl is 3: 1, give M + 2)