Mass Spectrometry From here To here Molecular Weight

- Slides: 22

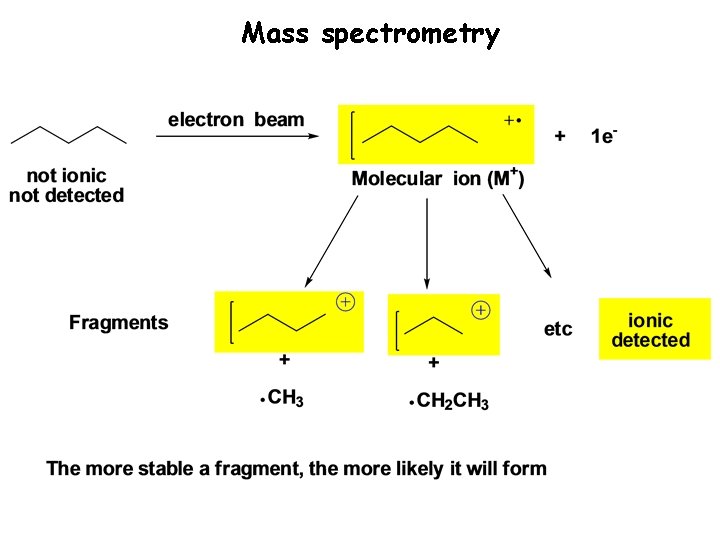
Mass Spectrometry From here… To here! Molecular Weight: 70 amu Molecular Formula: C 5 H 10 Detection of ionic species to determine the molecular weight of and obtain structural information on a molecule

Mass spectrometry

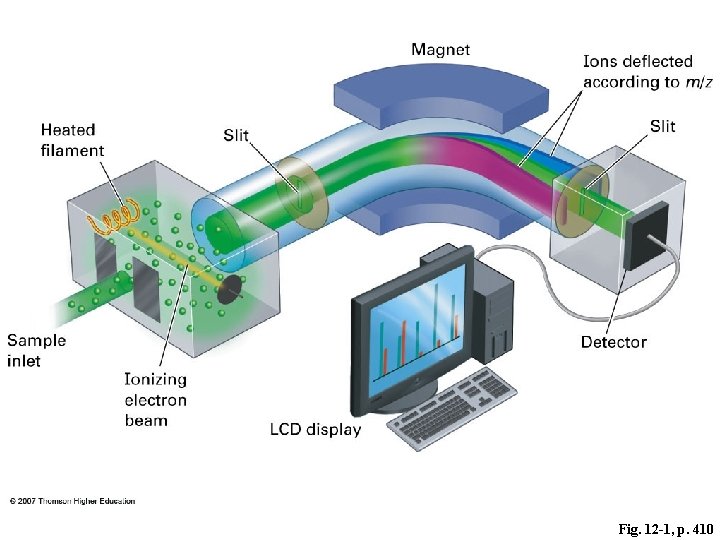
Fig. 12 -1, p. 410

Gas Chromatography – Mass Spectromety (GC-MS) Liquid Chromatography – Mass Spectromety (LC-MS)

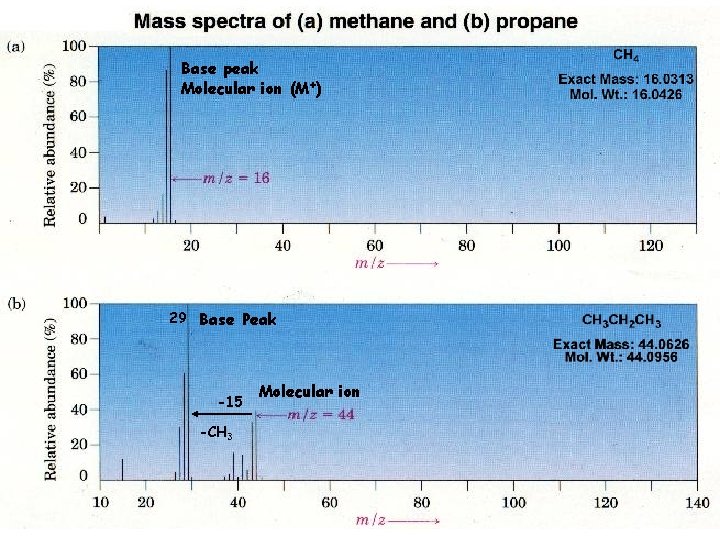
Base peak Molecular ion (M+) 29 Base Peak -15 -CH 3 Molecular ion

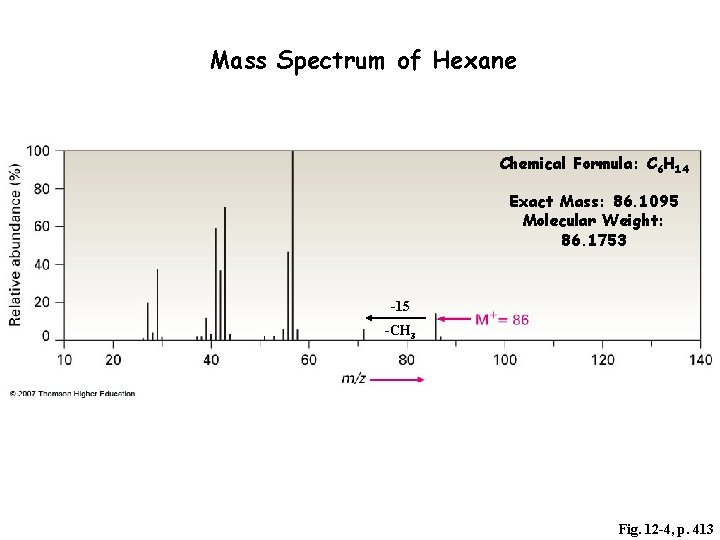
Mass Spectrum of Hexane Chemical Formula: C 6 H 14 Exact Mass: 86. 1095 Molecular Weight: 86. 1753 -15 -CH 3 Fig. 12 -4, p. 413

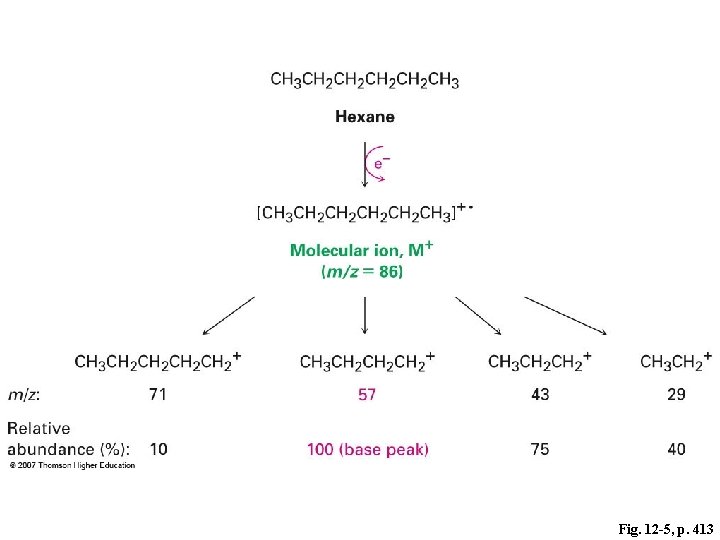
Fig. 12 -5, p. 413

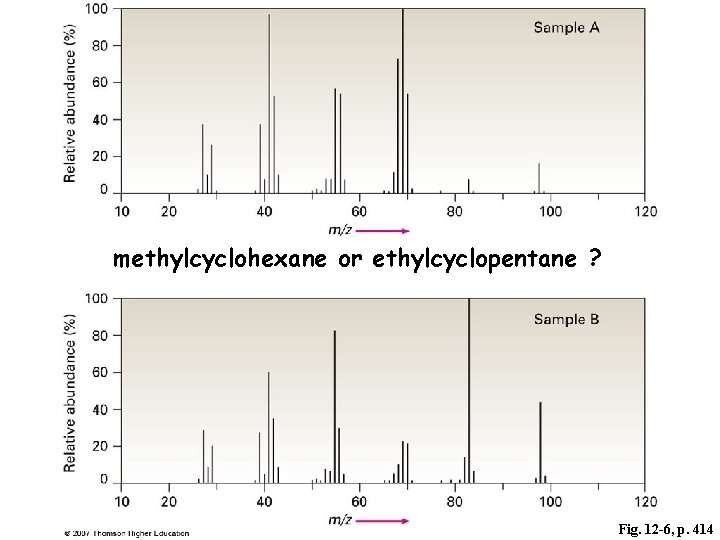
methylcyclohexane or ethylcyclopentane ? Fig. 12 -6, p. 414

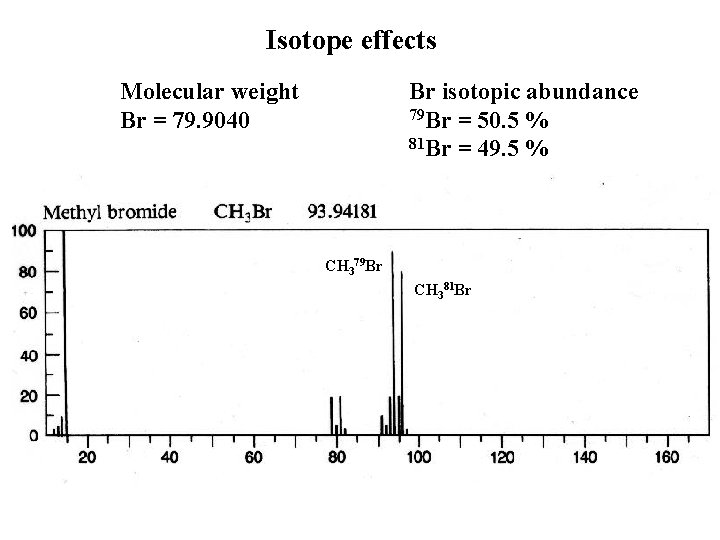
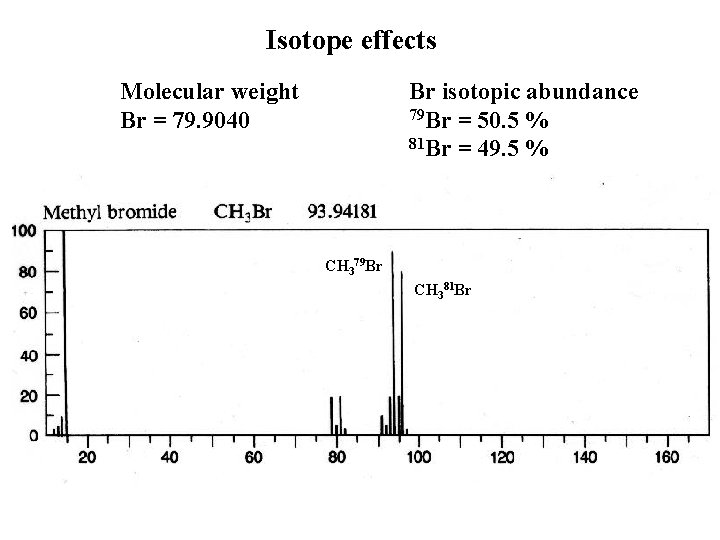
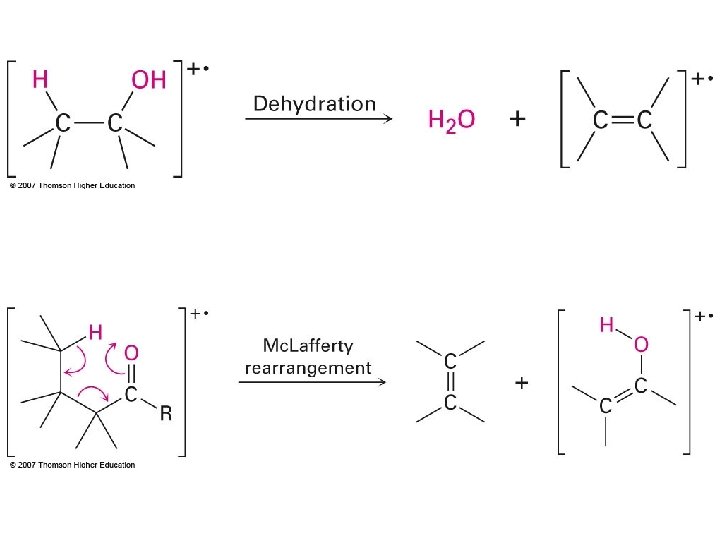
Isotope effects Molecular weight Br = 79. 9040 Br isotopic abundance 79 Br = 50. 5 % 81 Br = 49. 5 % CH 379 Br CH 381 Br

Isotope effects Molecular weight Br = 79. 9040 Br isotopic abundance 79 Br = 50. 5 % 81 Br = 49. 5 % CH 379 Br CH 381 Br

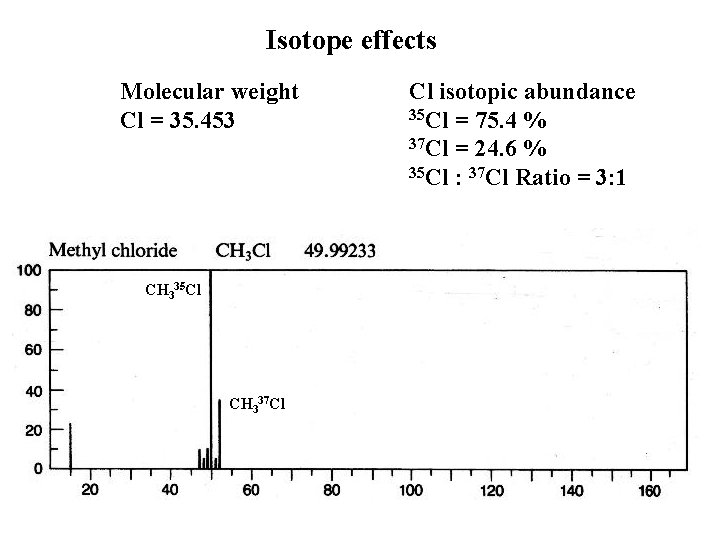
Isotope effects Molecular weight Cl = 35. 453 CH 335 Cl CH 337 Cl Cl isotopic abundance 35 Cl = 75. 4 % 37 Cl = 24. 6 % 35 Cl : 37 Cl Ratio = 3: 1

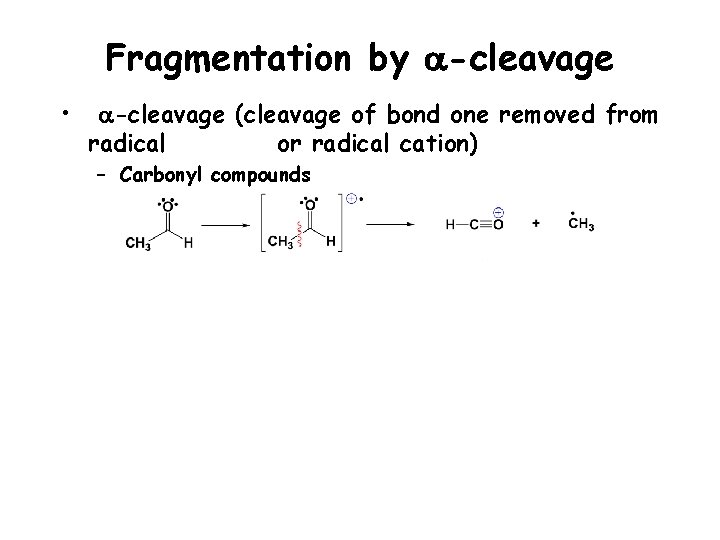
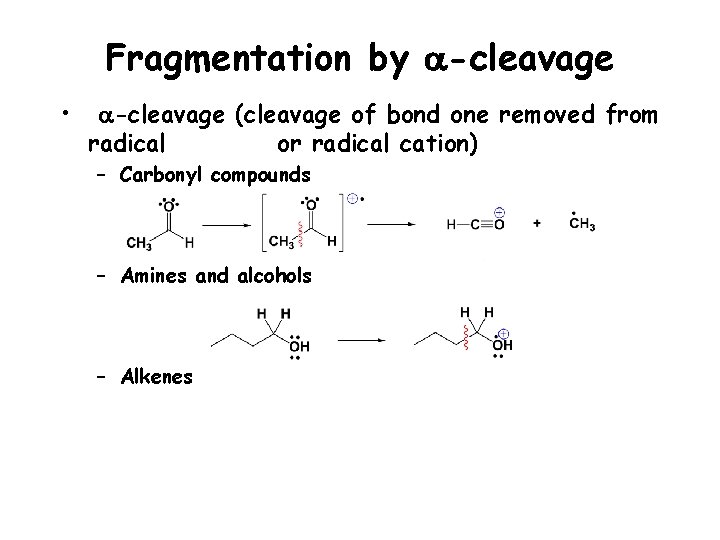
Fragmentation by a-cleavage • a-cleavage (cleavage of bond one removed from radical or radical cation) – Carbonyl compounds

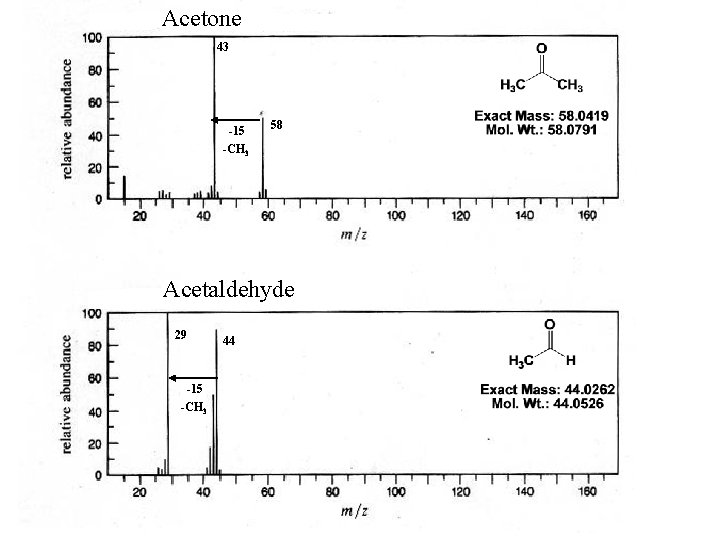
Acetone 43 -15 58 -CH 3 Acetaldehyde 29 -15 -CH 3 44

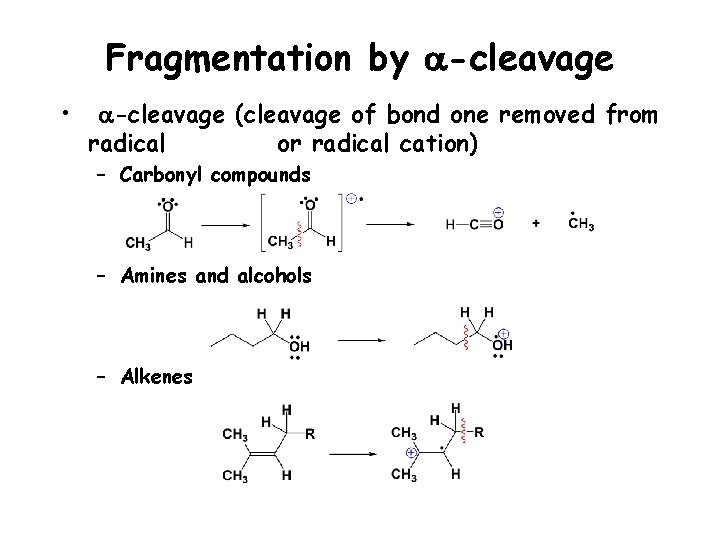
Fragmentation by a-cleavage • a-cleavage (cleavage of bond one removed from radical or radical cation) – Carbonyl compounds – Amines and alcohols – Alkenes

Fragmentation by a-cleavage • a-cleavage (cleavage of bond one removed from radical or radical cation) – Carbonyl compounds – Amines and alcohols – Alkenes

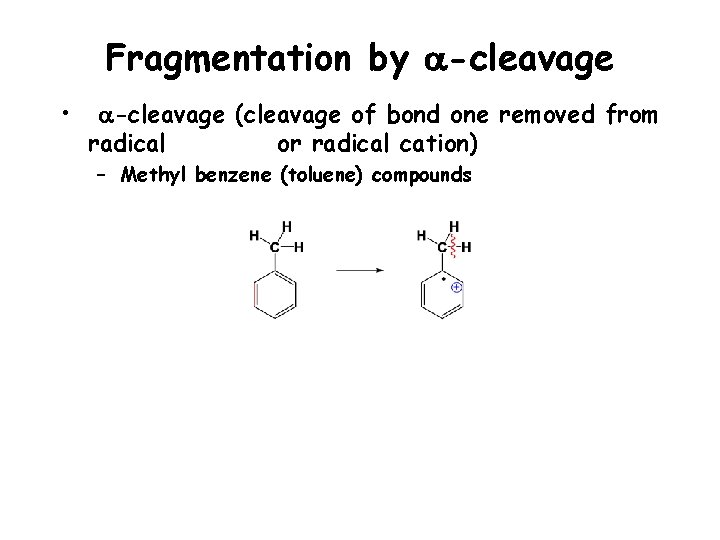
Fragmentation by a-cleavage • a-cleavage (cleavage of bond one removed from radical or radical cation) – Methyl benzene (toluene) compounds


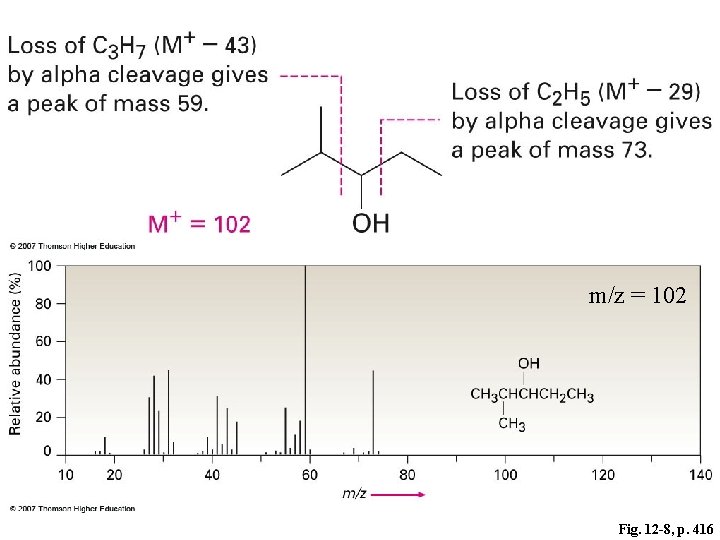
m/z = 102 Fig. 12 -8, p. 416

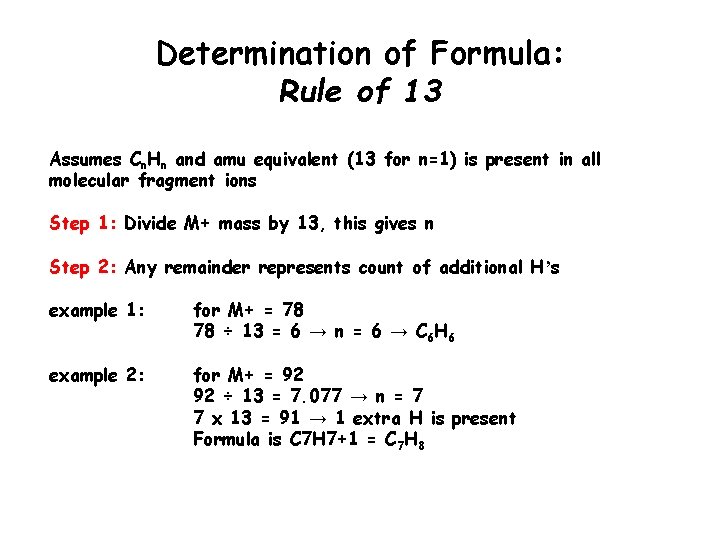
Determination of Formula: Rule of 13 Assumes Cn. Hn and amu equivalent (13 for n=1) is present in all molecular fragment ions Step 1: Divide M+ mass by 13, this gives n Step 2: Any remainder represents count of additional Hʼs example 1: for M+ = 78 78 ÷ 13 = 6 → n = 6 → C 6 H 6 example 2: for M+ = 92 92 ÷ 13 = 7. 077 → n = 7 7 x 13 = 91 → 1 extra H is present Formula is C 7 H 7+1 = C 7 H 8

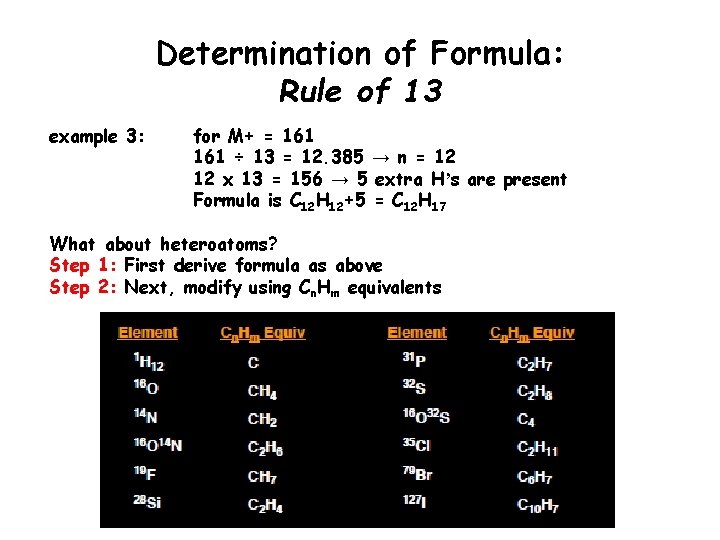
Determination of Formula: Rule of 13 example 3: for M+ = 161 ÷ 13 = 12. 385 → n = 12 12 x 13 = 156 → 5 extra Hʼs are present Formula is C 12 H 12+5 = C 12 H 17 What about heteroatoms? Step 1: First derive formula as above Step 2: Next, modify using Cn. Hm equivalents

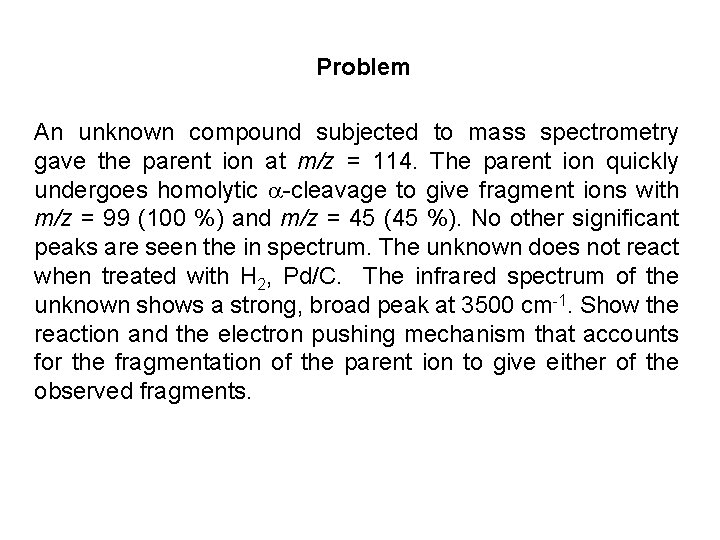
Problem An unknown compound subjected to mass spectrometry gave the parent ion at m/z = 114. The parent ion quickly undergoes homolytic -cleavage to give fragment ions with m/z = 99 (100 %) and m/z = 45 (45 %). No other significant peaks are seen the in spectrum. The unknown does not react when treated with H 2, Pd/C. The infrared spectrum of the unknown shows a strong, broad peak at 3500 cm-1. Show the reaction and the electron pushing mechanism that accounts for the fragmentation of the parent ion to give either of the observed fragments.

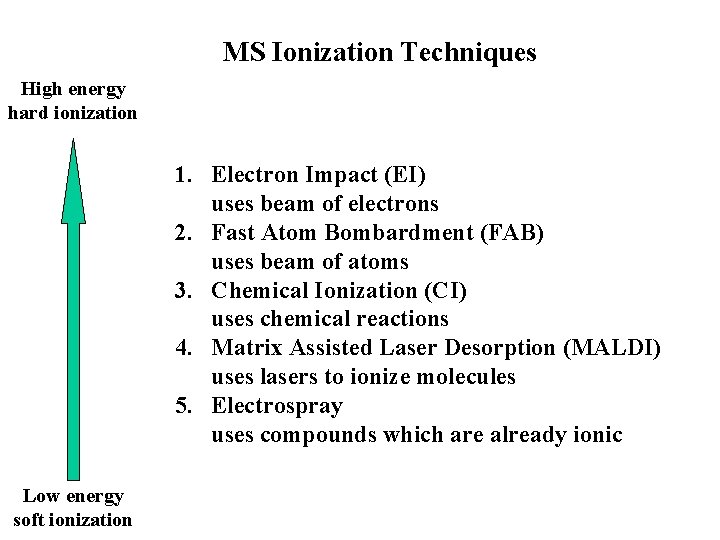
MS Ionization Techniques High energy hard ionization 1. Electron Impact (EI) uses beam of electrons 2. Fast Atom Bombardment (FAB) uses beam of atoms 3. Chemical Ionization (CI) uses chemical reactions 4. Matrix Assisted Laser Desorption (MALDI) uses lasers to ionize molecules 5. Electrospray uses compounds which are already ionic Low energy soft ionization