Mass Relationships in Chemical Reactions Atomic Mass 0





![Example Urea [(NH 2)2 CO] is prepared by reacting ammonia with carbon dioxide in Example Urea [(NH 2)2 CO] is prepared by reacting ammonia with carbon dioxide in](https://slidetodoc.com/presentation_image_h/34409540666d64496464df9096b37700/image-24.jpg)

- Slides: 26


Mass Relationships in Chemical Reactions

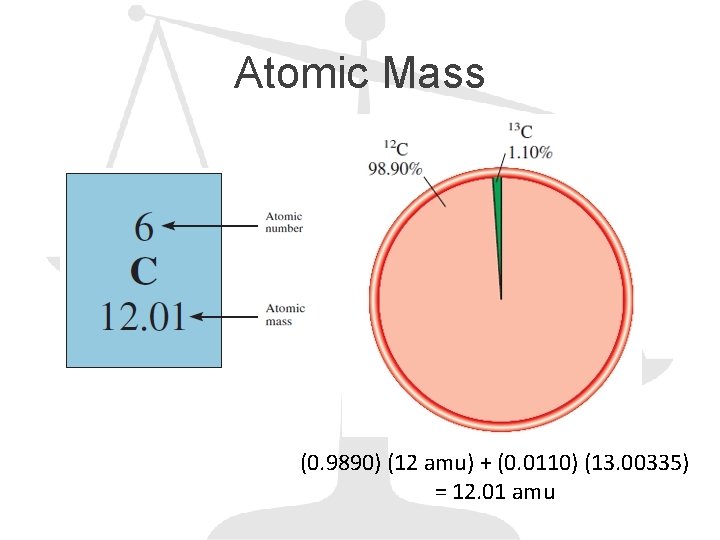
Atomic Mass (0. 9890) (12 amu) + (0. 0110) (13. 00335) = 12. 01 amu

Mole Avogadro’s number: 6. 022 x 1023 (atoms, molecules, particles) Molar mass is numerically the same as atomic mass of an element

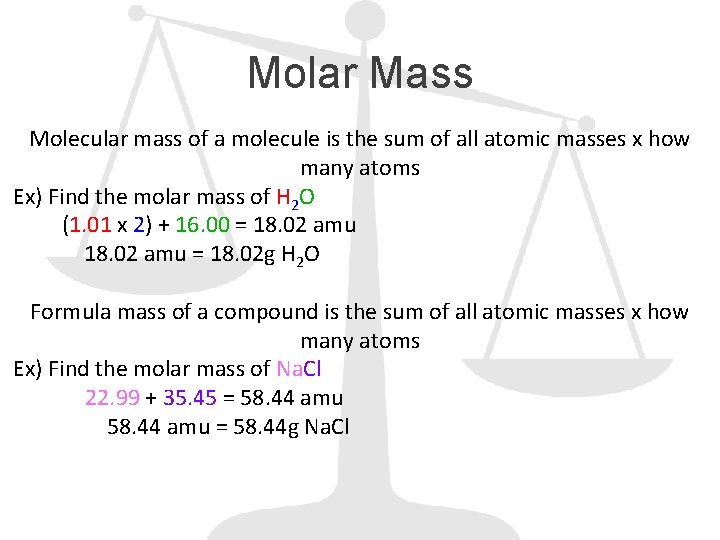
Molar Mass Molecular mass of a molecule is the sum of all atomic masses x how many atoms Ex) Find the molar mass of H 2 O (1. 01 x 2) + 16. 00 = 18. 02 amu = 18. 02 g H 2 O Formula mass of a compound is the sum of all atomic masses x how many atoms Ex) Find the molar mass of Na. Cl 22. 99 + 35. 45 = 58. 44 amu = 58. 44 g Na. Cl

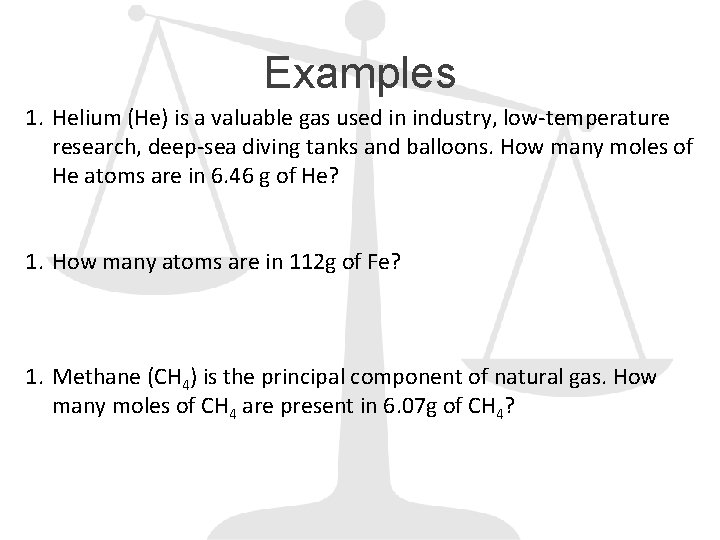
Examples 1. Helium (He) is a valuable gas used in industry, low-temperature research, deep-sea diving tanks and balloons. How many moles of He atoms are in 6. 46 g of He? 1. How many atoms are in 112 g of Fe? 1. Methane (CH 4) is the principal component of natural gas. How many moles of CH 4 are present in 6. 07 g of CH 4?

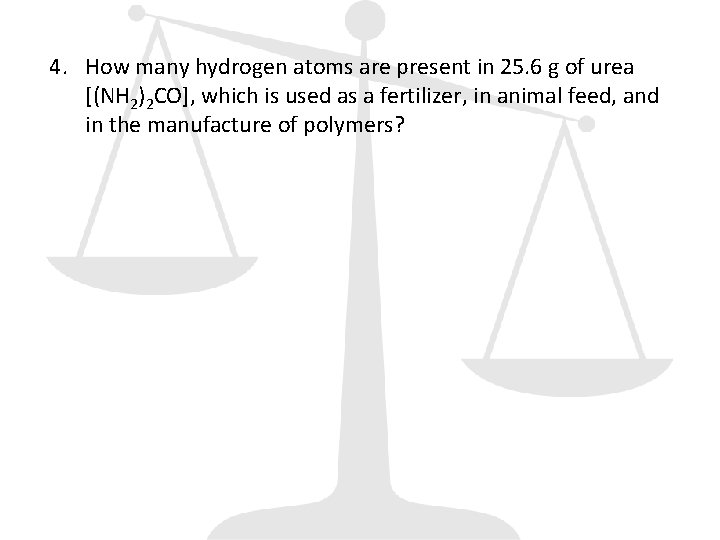
4. How many hydrogen atoms are present in 25. 6 g of urea [(NH 2)2 CO], which is used as a fertilizer, in animal feed, and in the manufacture of polymers?

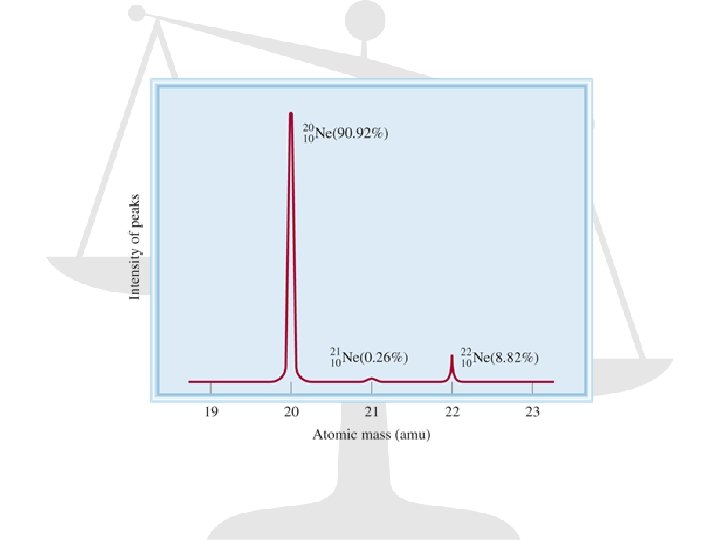
Mass Spectrometry


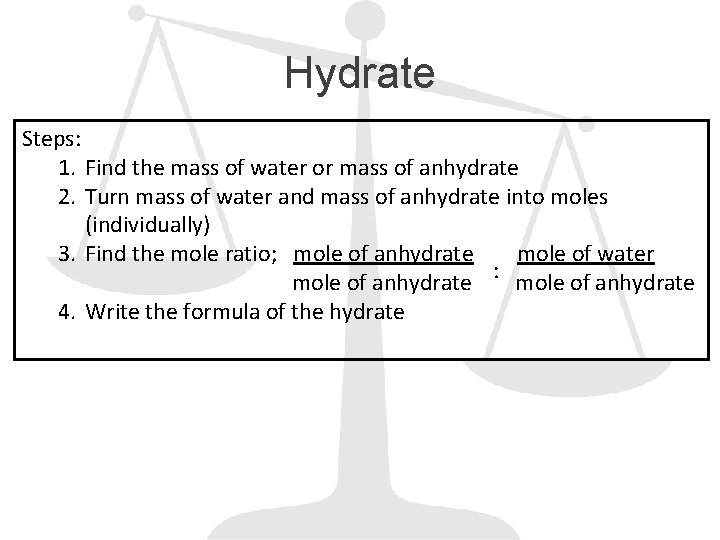
Hydrate Steps: 1. Find the mass of water or mass of anhydrate 2. Turn mass of water and mass of anhydrate into moles (individually) 3. Find the mole ratio; mole of anhydrate mole of water : mole of anhydrate 4. Write the formula of the hydrate

Example A calcium chlorite hydrate has a mass of 4. 72 g. After heating for several minutes the mass of the anhydrate is found to be 3. 56 g. Use this information to determine the formula for the hydrate.

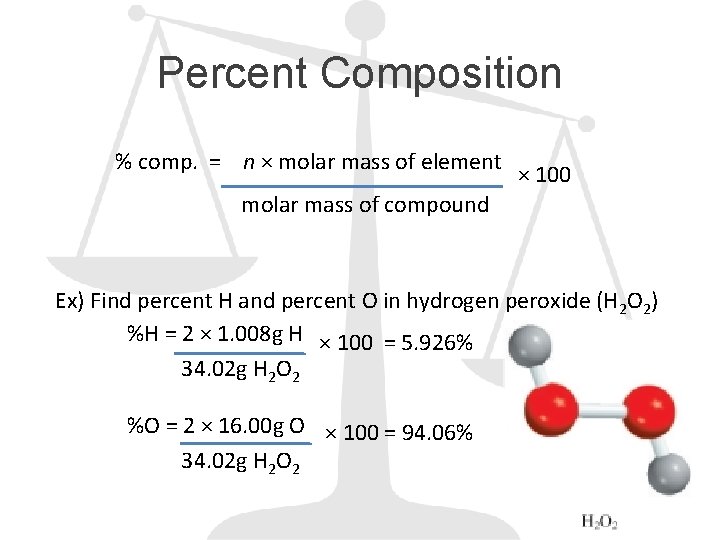
Percent Composition % comp. = n × molar mass of element × 100 molar mass of compound Ex) Find percent H and percent O in hydrogen peroxide (H 2 O 2) %H = 2 × 1. 008 g H × 100 = 5. 926% 34. 02 g H 2 O 2 %O = 2 × 16. 00 g O × 100 = 94. 06% 34. 02 g H 2 O 2

Empirical Formula Steps: 1. Percent to mass 2. Mass to moles 3. Divide by small 4. Multiply ‘til whole

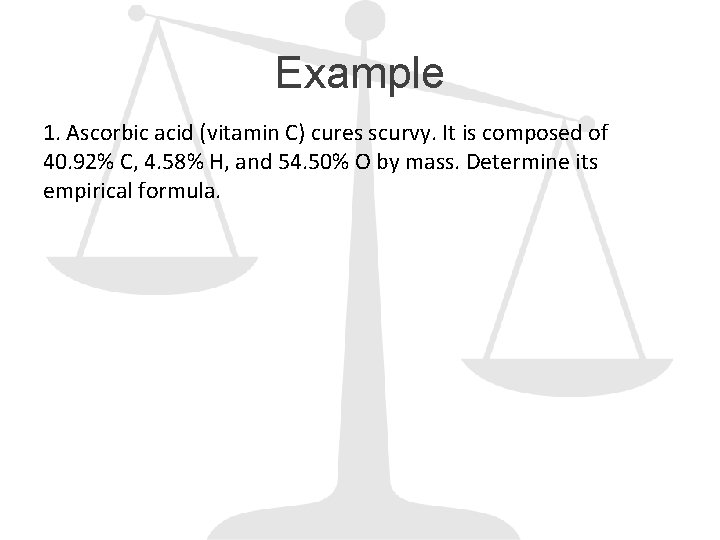
Example 1. Ascorbic acid (vitamin C) cures scurvy. It is composed of 40. 92% C, 4. 58% H, and 54. 50% O by mass. Determine its empirical formula.


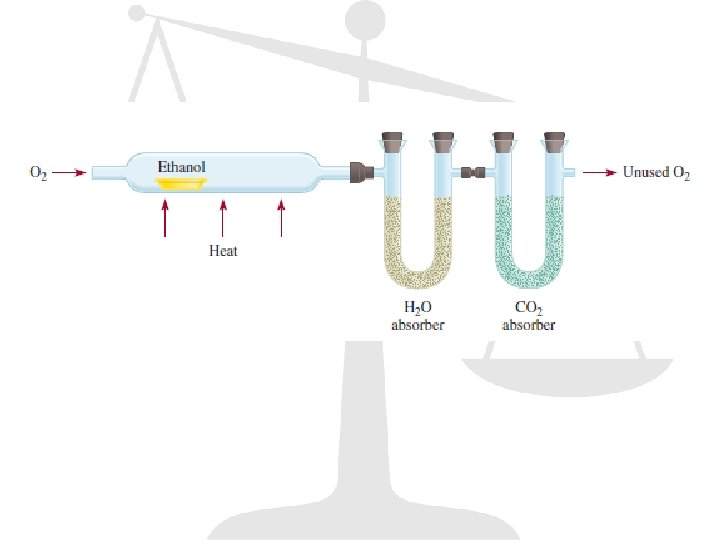
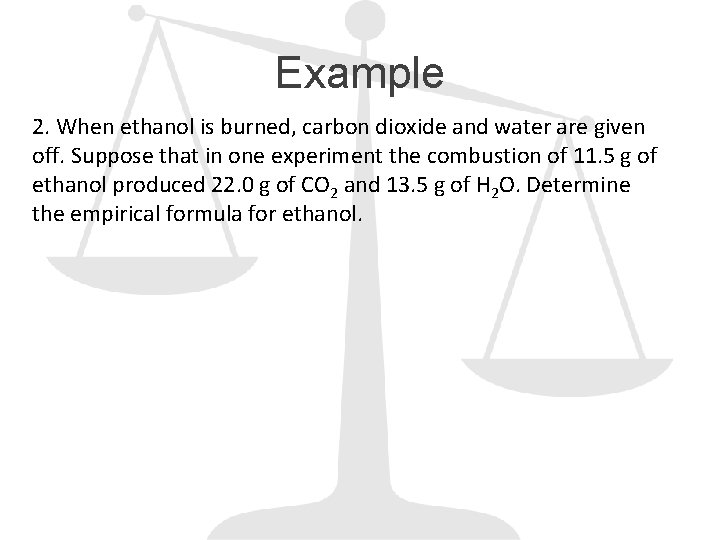
Example 2. When ethanol is burned, carbon dioxide and water are given off. Suppose that in one experiment the combustion of 11. 5 g of ethanol produced 22. 0 g of CO 2 and 13. 5 g of H 2 O. Determine the empirical formula for ethanol.

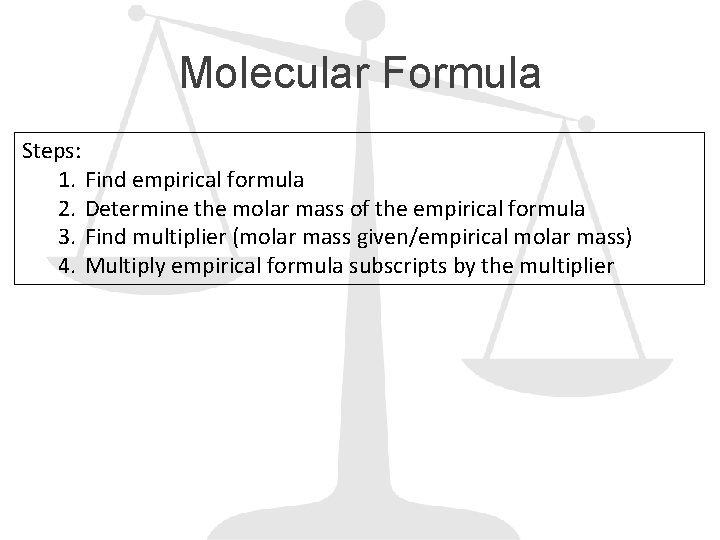
Molecular Formula Steps: 1. Find empirical formula 2. Determine the molar mass of the empirical formula 3. Find multiplier (molar mass given/empirical molar mass) 4. Multiply empirical formula subscripts by the multiplier

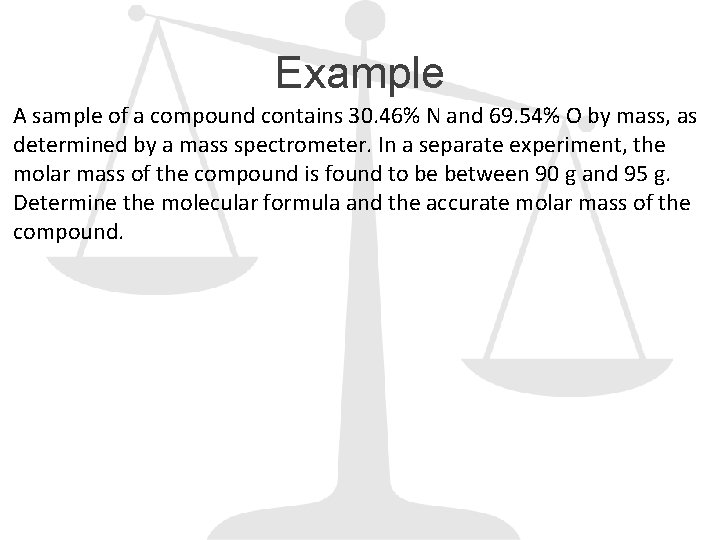
Example A sample of a compound contains 30. 46% N and 69. 54% O by mass, as determined by a mass spectrometer. In a separate experiment, the molar mass of the compound is found to be between 90 g and 95 g. Determine the molecular formula and the accurate molar mass of the compound.

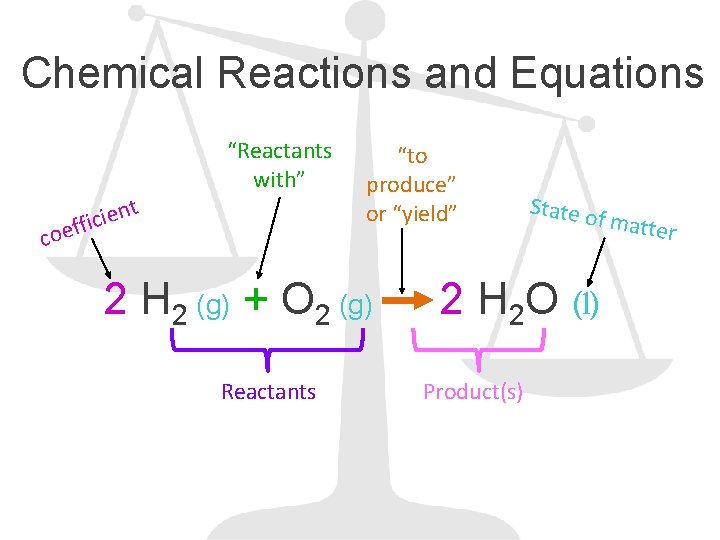
Chemical Reactions and Equations t n e i effic “Reactants with” co “to produce” or “yield” 2 H 2 (g) + O 2 (g) Reactants State o f matte 2 H 2 O (l) Product(s) r

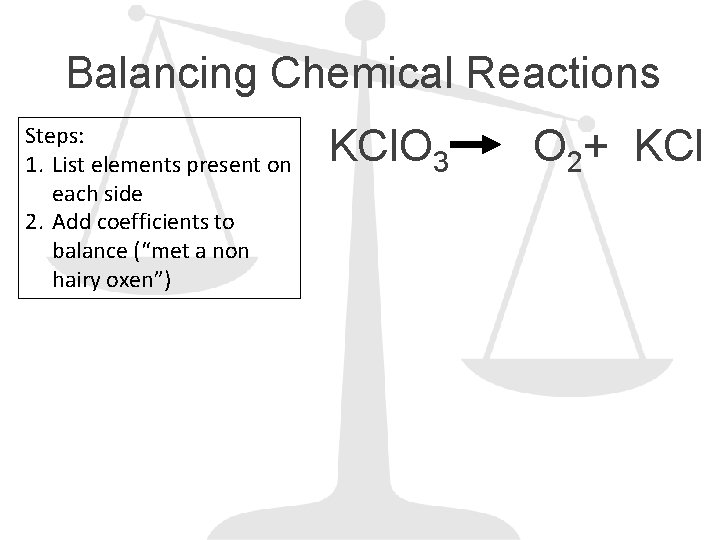
Balancing Chemical Reactions Steps: 1. List elements present on each side 2. Add coefficients to balance (“met a non hairy oxen”) KCl. O 3 O 2+ KCl

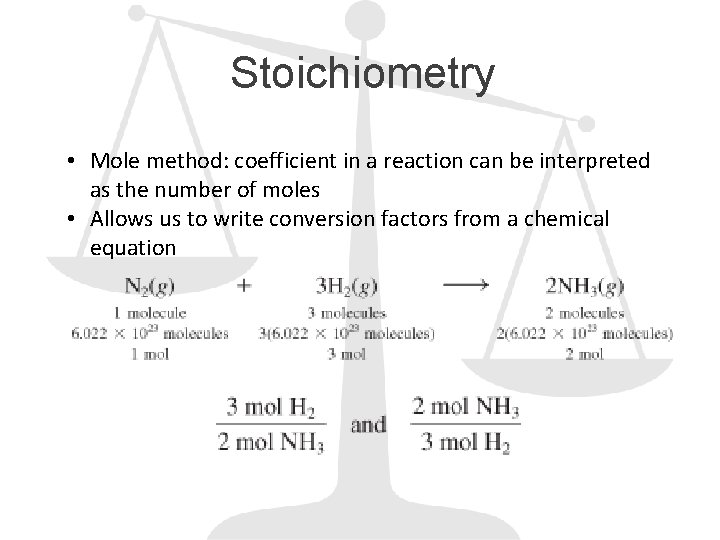
Stoichiometry • Mole method: coefficient in a reaction can be interpreted as the number of moles • Allows us to write conversion factors from a chemical equation

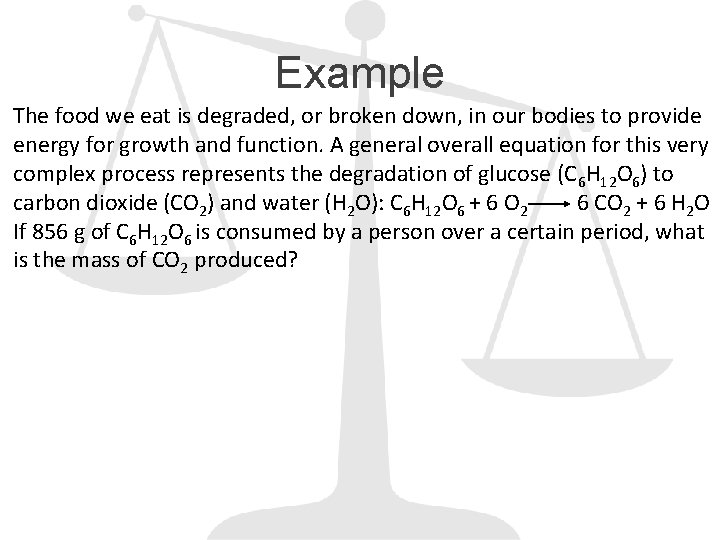
Example The food we eat is degraded, or broken down, in our bodies to provide energy for growth and function. A general overall equation for this very complex process represents the degradation of glucose (C 6 H 12 O 6) to carbon dioxide (CO 2) and water (H 2 O): C 6 H 12 O 6 + 6 O 2 6 CO 2 + 6 H 2 O If 856 g of C 6 H 12 O 6 is consumed by a person over a certain period, what is the mass of CO 2 produced?

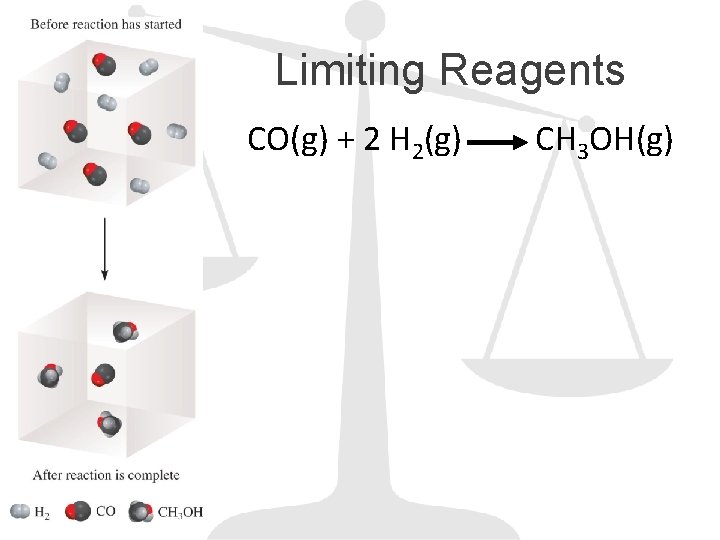
Limiting Reagents CO(g) + 2 H 2(g) CH 3 OH(g)
![Example Urea NH 22 CO is prepared by reacting ammonia with carbon dioxide in Example Urea [(NH 2)2 CO] is prepared by reacting ammonia with carbon dioxide in](https://slidetodoc.com/presentation_image_h/34409540666d64496464df9096b37700/image-24.jpg)
Example Urea [(NH 2)2 CO] is prepared by reacting ammonia with carbon dioxide in the following process: 2 NH 3(g) + CO 2(g) (NH 2)2 CO(aq) + H 2 O(l) In one process, 637. 2 g of NH 3 are treated with 1142 g CO 2. Which of the reactants is the limiting reagent? Calculate the mass of [(NH 2)2 CO] formed. How much excess reagent (in grams) is left at the end of the reaction?

Reaction Yield

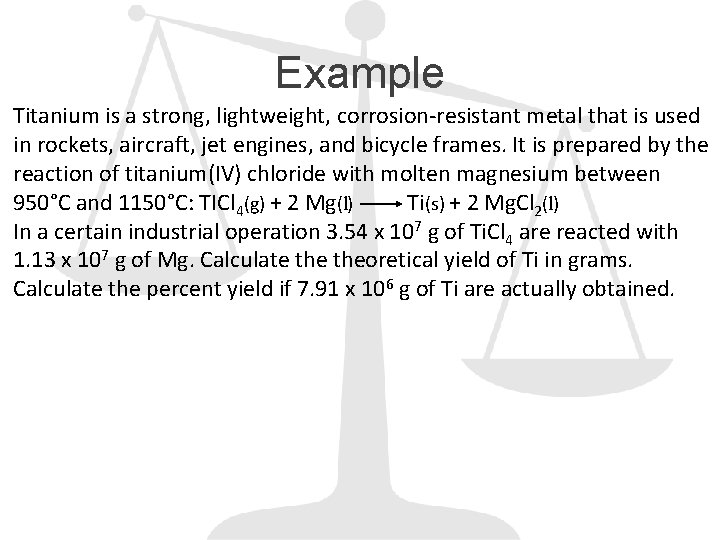
Example Titanium is a strong, lightweight, corrosion-resistant metal that is used in rockets, aircraft, jet engines, and bicycle frames. It is prepared by the reaction of titanium(IV) chloride with molten magnesium between 950°C and 1150°C: TICl 4(g) + 2 Mg(l) Ti(s) + 2 Mg. Cl 2(l) In a certain industrial operation 3. 54 x 107 g of Ti. Cl 4 are reacted with 1. 13 x 107 g of Mg. Calculate theoretical yield of Ti in grams. Calculate the percent yield if 7. 91 x 106 g of Ti are actually obtained.
 Section 2 classifying chemical reactions
Section 2 classifying chemical reactions Section 2 classifying chemical reactions worksheet answers
Section 2 classifying chemical reactions worksheet answers Mass relationships in chemical reactions
Mass relationships in chemical reactions Relative formula mass of hcl
Relative formula mass of hcl How do you calculate atomic mass
How do you calculate atomic mass Chemical reactions section 3 reactions in aqueous solutions
Chemical reactions section 3 reactions in aqueous solutions Stoichiometry island diagram
Stoichiometry island diagram Chemical reactions section 1 chemical changes
Chemical reactions section 1 chemical changes Are kc and kp equal
Are kc and kp equal Atomic mass and atomic number difference
Atomic mass and atomic number difference Law of conservation of mass
Law of conservation of mass What is mass number
What is mass number Mole bridge chemistry
Mole bridge chemistry Atomic mass vs molar mass
Atomic mass vs molar mass What are redox reactions examples
What are redox reactions examples Unit 5 chemical reactions answers
Unit 5 chemical reactions answers Chemistry periodic trends
Chemistry periodic trends Ionic radius trend
Ionic radius trend Atomic number vs atomic radius
Atomic number vs atomic radius Examples of chemical change
Examples of chemical change Balancing redox reactions
Balancing redox reactions How to identify types of chemical reactions
How to identify types of chemical reactions Types of reactions chemistry
Types of reactions chemistry Reaction type
Reaction type Predicting products of chemical reactions
Predicting products of chemical reactions 4 types of chemical reactions
4 types of chemical reactions Non examples of chemical reactions
Non examples of chemical reactions


















































