Mass Measurements In Chemistry Calculating Formula Weight I






- Slides: 13
Mass Measurements In Chemistry Calculating Formula Weight

• I CAN calculate the formula weight (molar mass) of a compound from it’s formula.

We’ve talked about the mass of atoms in terms of one or two…. but these masses are very small. To be practical for laboratory work, a standard of measure had to be derived so that mass quantities could be easily measured. To that end, scientists created a unit called the ATOMIC MASS UNIT used to measure the mass of subatomic particles.

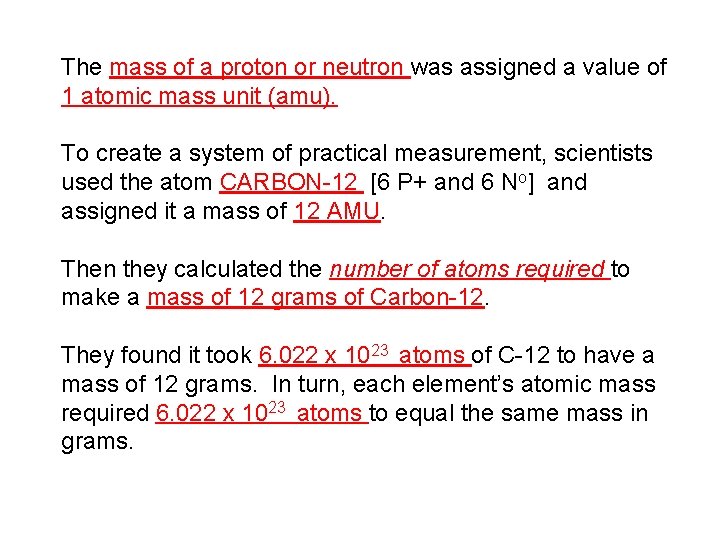
The mass of a proton or neutron was assigned a value of 1 atomic mass unit (amu). To create a system of practical measurement, scientists used the atom CARBON-12 [6 P+ and 6 No] and assigned it a mass of 12 AMU. Then they calculated the number of atoms required to make a mass of 12 grams of Carbon-12. They found it took 6. 022 x 1023 atoms of C-12 to have a mass of 12 grams. In turn, each element’s atomic mass required 6. 022 x 1023 atoms to equal the same mass in grams.

FOR EXAMPLE 1 atom of Oxygen 16 weighs 16 AMU. 6. 022 x 1023 atoms of O-16 weighs 16 grams! This number became very important in chemistry is known as: Avagadro’s Number

The quantity of matter containing Avagadro’s Number of particles, such as atoms, molecules or formula units, is known as a: Mole When working in a lab setting, the MOLE is used to determine quantities of chemicals.

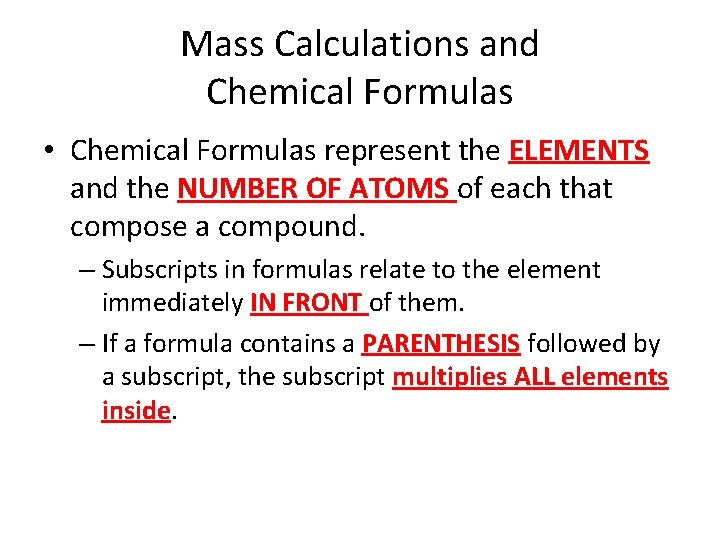
Mass Calculations and Chemical Formulas • Chemical Formulas represent the ELEMENTS and the NUMBER OF ATOMS of each that compose a compound. – Subscripts in formulas relate to the element immediately IN FRONT of them. – If a formula contains a PARENTHESIS followed by a subscript, the subscript multiplies ALL elements inside.

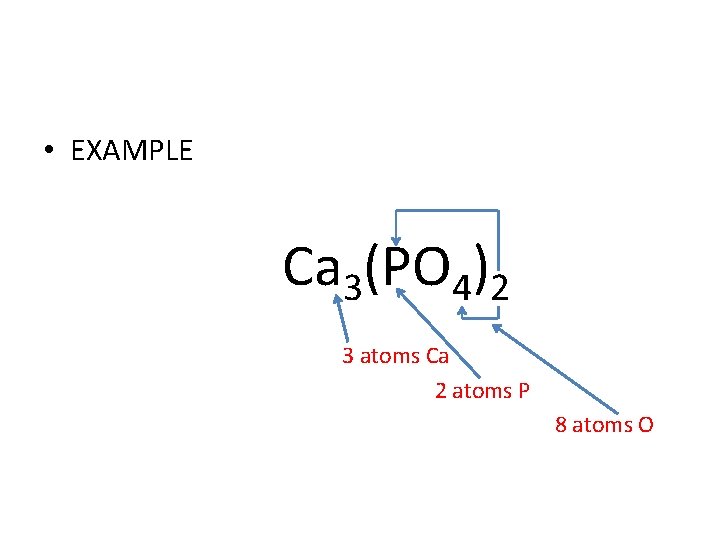
• EXAMPLE Ca 3(PO 4)2 3 atoms Ca 2 atoms P 8 atoms O

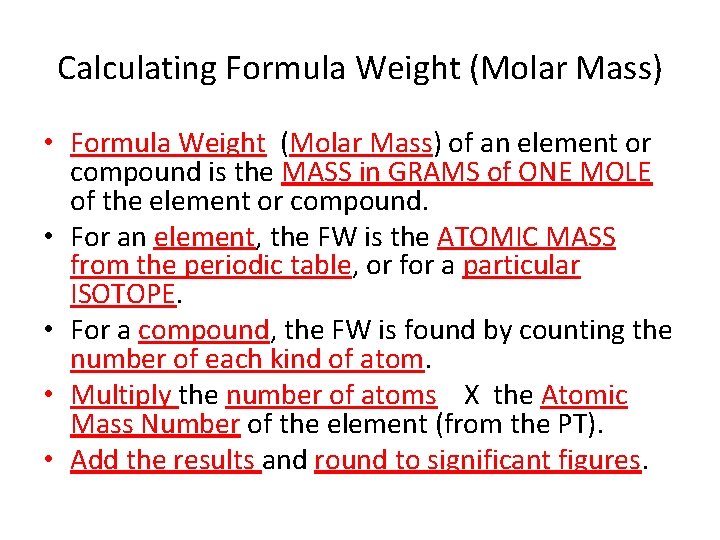
Calculating Formula Weight (Molar Mass) • Formula Weight (Molar Mass) of an element or compound is the MASS in GRAMS of ONE MOLE of the element or compound. • For an element, the FW is the ATOMIC MASS from the periodic table, or for a particular ISOTOPE. • For a compound, the FW is found by counting the number of each kind of atom. • Multiply the number of atoms X the Atomic Mass Number of the element (from the PT). • Add the results and round to significant figures.

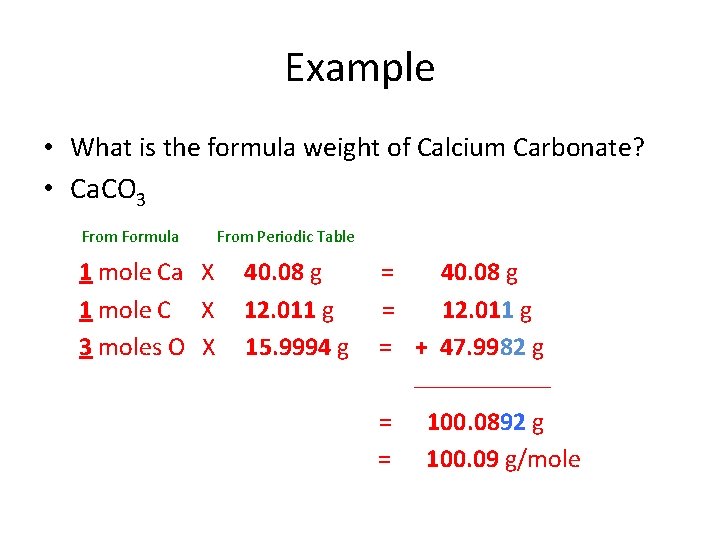
Example • What is the formula weight of Calcium Carbonate? • Ca. CO 3 From Formula 1 mole Ca X 1 mole C X 3 moles O X From Periodic Table 40. 08 g 12. 011 g 15. 9994 g = 40. 08 g = 12. 011 g = + 47. 9982 g ________ = = 100. 0892 g 100. 09 g/mole

Hydrates • Certain ionic compounds trap water molecules in their structure when they form. • These are known as HYDRATES. • Hydrates are written in this manner: • Compound Formula Dot Number of Water Molecules • Cu. SO 4 5 H 2 O • Hydrates are named as: • Compound Name, a Prefix for the number of water molecules, then the word Hydrate. • When calculating the formula weight of a hydrate, just include the MASS OF THE NUMBER OF WATER MOLECULES in the total.

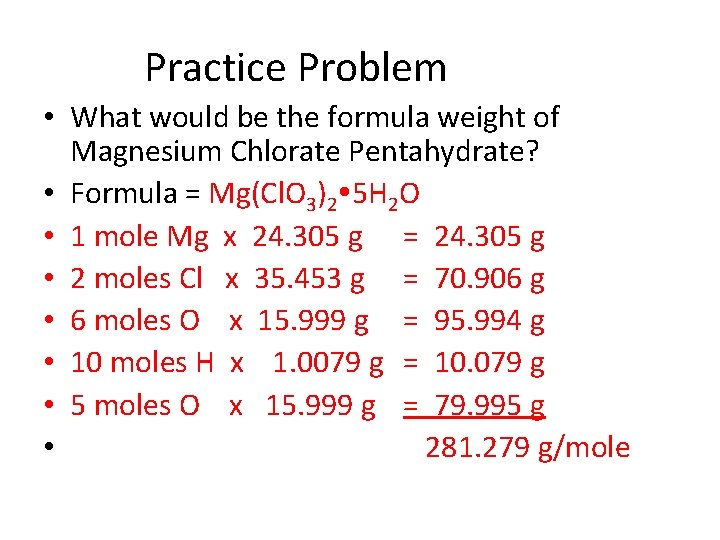
Practice Problem • What would be the formula weight of Magnesium Chlorate Pentahydrate? • Formula = Mg(Cl. O 3)2 5 H 2 O • 1 mole Mg x 24. 305 g = 24. 305 g • 2 moles Cl x 35. 453 g = 70. 906 g • 6 moles O x 15. 999 g = 95. 994 g • 10 moles H x 1. 0079 g = 10. 079 g • 5 moles O x 15. 999 g = 79. 995 g • 281. 279 g/mole

PRACTICE PROBLEMS • Complete the following FORMULA WEIGHT (MOLAR MASS) calculations ON YOUR OWN PAPER! • 1. Find the FORMULA WEIGHT of Aluminum Sulfate, Al 2(SO 4)3. • 2. What is the MOLAR MASS of Glucose, C 6 H 12 O 6? • 3. Find the FORMULA WEIGHT of: Sodium Potassium Permanganate Tetrahydrate Na. K(Mn. O 4)2 4 H 2 O • 4. What is the MOLAR MASS of Ammonium Phosphate Dihydrate.