Mass Energy E mc 2 Energy and mass




- Slides: 11
Mass Energy • E = mc 2 • Energy and mass are equivalent • C = 3 x 108 m/s. • C is a big number and its squared! So even if m is small, E is big. • A small mass, converted to energy, gives a lot of energy!

Example

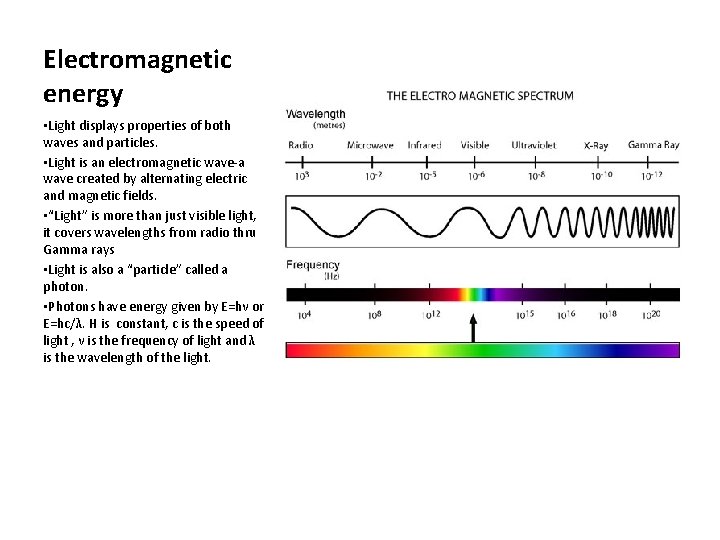
Electromagnetic energy • Light displays properties of both waves and particles. • Light is an electromagnetic wave-a wave created by alternating electric and magnetic fields. • “Light” is more than just visible light, it covers wavelengths from radio thru Gamma rays • Light is also a “particle” called a photon. • Photons have energy given by E=hν or E=hc/λ. H is constant, c is the speed of light , ν is the frequency of light and λ is the wavelength of the light.

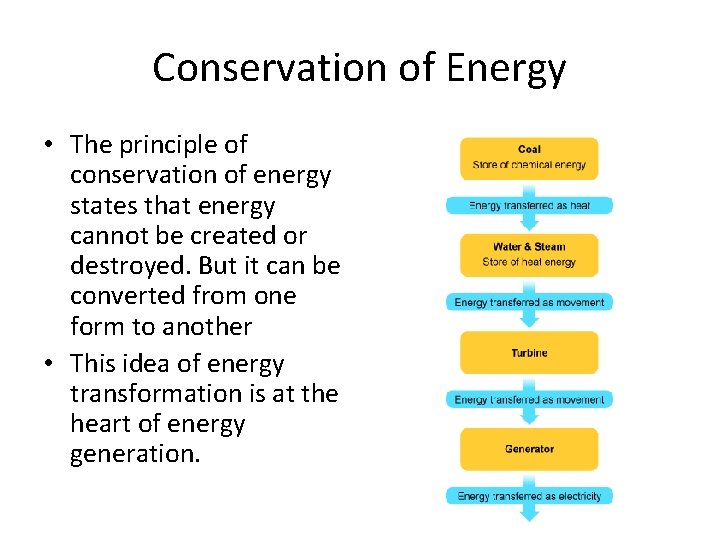
Conservation of Energy • The principle of conservation of energy states that energy cannot be created or destroyed. But it can be converted from one form to another • This idea of energy transformation is at the heart of energy generation.

Energy Sources renewable vs nonrewnewable • Renewable – can’t be exhausted • Solar • Geo-thermal • Tidal • Wind • Hydro • Non-renewable-can be exhausted • Fossil fuels (oil, coal etc) • Nuclear

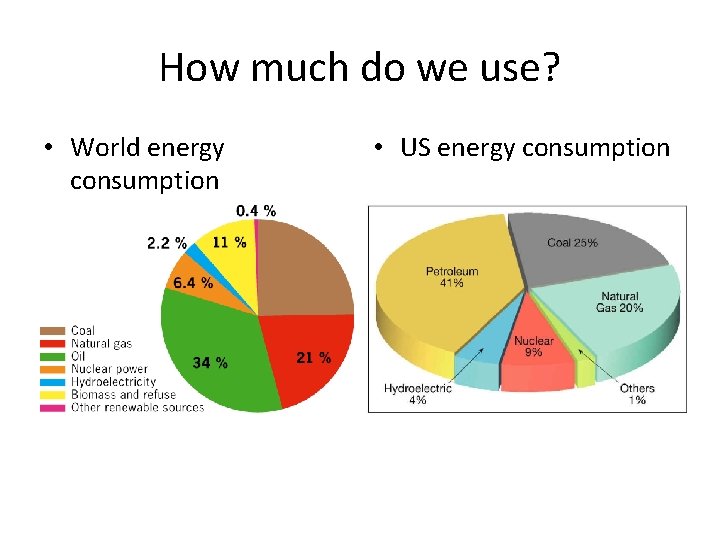
How much do we use? • World energy consumption • US energy consumption

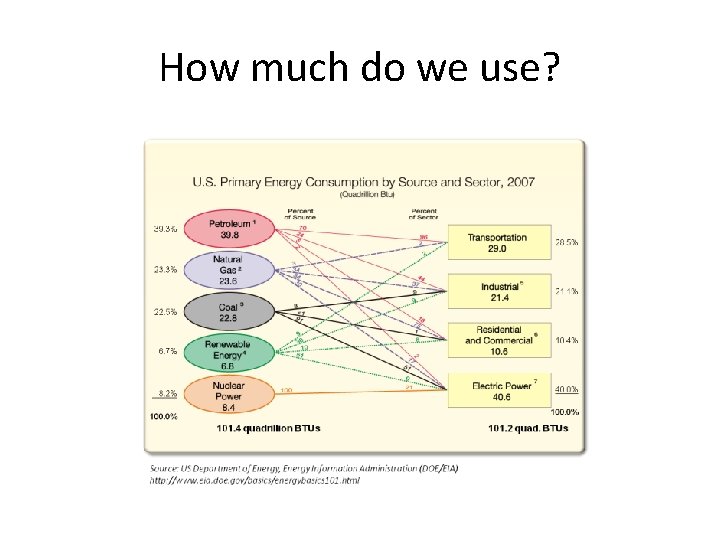
How much do we use?

How much do we use? • Almost 95% of the energy we use comes from non-renewble energy sources! • One of these days we will run out, and then what? • What are some short and long term answers to this question?

Fossil fuels

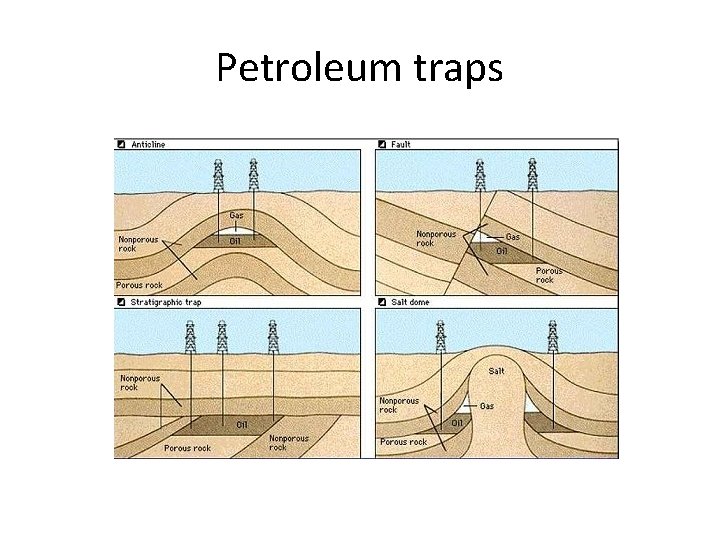
FOSSIL FUELS • Carbon or hydrocarbons (a compound made of hydrogen and carbon) found in the earth’s crust • Formed from the bacterial decay of plant and animal life in ancient (a few hundred million years ago) seas. • The decomposing material was covered with mud and sediment • This increased the pressure and temperature on the material and deprived it of oxygen. • A variety of hydrocarbon molecules are created in solid, liquid and gas states. • The gas and liquid could travel through the porous rock and collect in geological traps (rock features that prevent further movement of the hydrocarbons).

Petroleum traps