Mass and the Mole Chemistry Section 11 2










- Slides: 18

Mass and the Mole Chemistry Section 11. 2

Objectives n n Relate the mass of an atom to the mass of a mole of atoms. Calculate the number of moles in a given mass of an element and the mass of a given number of moles of an element. Calculate the number of moles of an element when given the number of atoms of the element. Calculate the number of atoms of an element when given the number of moles of the element.

The Mass of the Mole n n Do a dozen limes have the same mass as a dozen eggs? No. The same is true for moles. One mol of Fe does not have the same mass as one mol of Ag.

Molar Mass n n This is IMPORTANT! Mass in grams of 1 mole of any pure substance is termed the molar mass. Molar mass of any element equals its atomic mass Units are g/mol

Molar Mass n For Example: n n n n Manganese What is Manganese’s molar mass? 54. 94 amu OR g/mol What does one mole of Manganese weigh? 54. 94 g How many atoms of Manganese are in one mol? 6. 022 x 1023

Molar Mass n n We can use the molar mass of every element as a conversion factor. Example: # of mol x # of grams = mass 1 mol

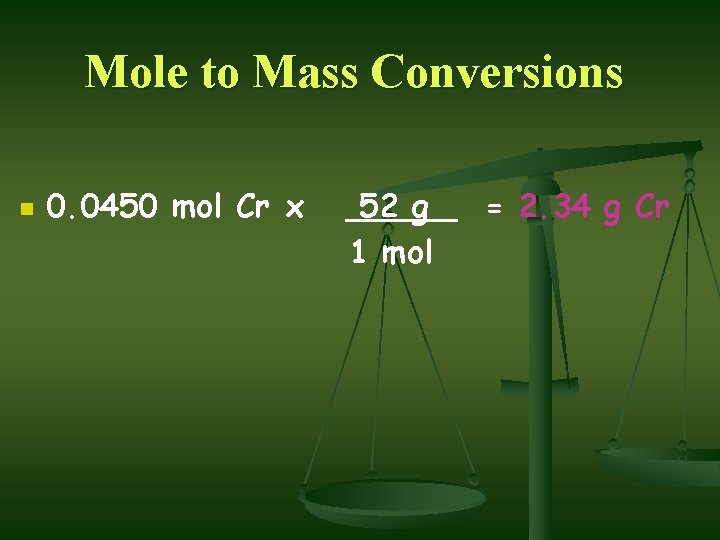
Mole to Mass Conversions n n n Chromium (Cr) is a transition element. Calculate the mass in grams of 0. 0450 moles of Cr. Always start with ? n n What is given in the problem! 0. 0450 mol Cr

Mole to Mass Conversions n n n Molar mass is the conversion factor 52. 00 g/1 mol Cr Put what you want to end up with on the top of the conversion factor.

Mole to Mass Conversions n 0. 0450 mol Cr x 52 g 1 mol = 2. 34 g Cr

Practice Problems n n Pg. 316 12

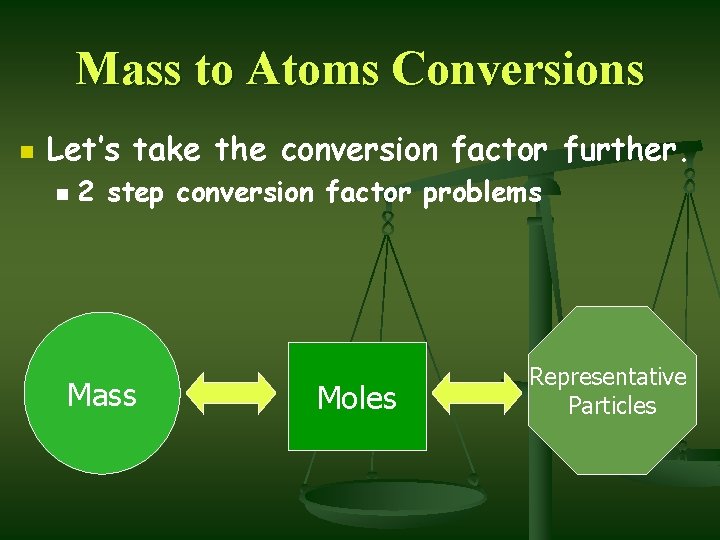
Mass to Atoms Conversions n Let’s take the conversion factor further. n 2 step conversion factor problems Mass Moles Representative Particles

Mass to Atoms Conversions n n If given the mass of an element, you can find the number of atoms in that mass. Example: How many atoms of gold are in a pure gold nugget having a mass of 25. 0 g?

Mass to Atoms Conversions n n n Always start with? What is given in the problem. 25. 0 g Au n Mass Moles Particles

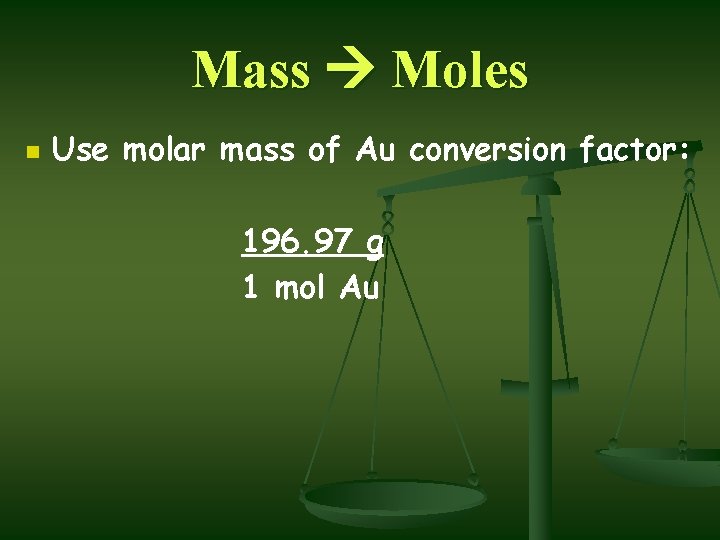
Mass Moles n Use molar mass of Au conversion factor: 196. 97 g 1 mol Au

Moles Particles n Avogadro’s conversion: 6. 022 x 1023 atoms 1 mol Au

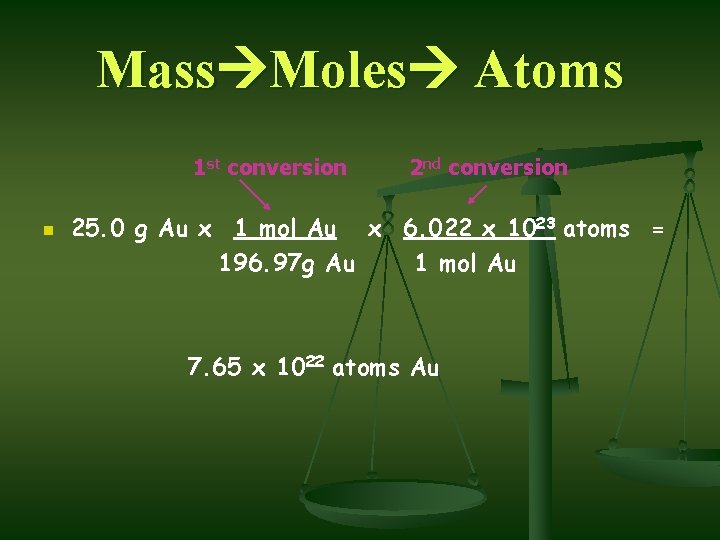
Mass Moles Atoms 1 st conversion n 2 nd conversion 25. 0 g Au x 1 mol Au x 6. 022 x 1023 atoms = 196. 97 g Au 1 mol Au 7. 65 x 1022 atoms Au

Practice Problems n n Pg 318 14

Homework n Molar mass worksheet