MASCC Guideline Nausea vomiting in advanced cancer PALLIATIVE





- Slides: 13

MASCC Guideline: Nausea & vomiting in advanced cancer PALLIATIVE CARE STUDY GROUP

Citation v Walsh D, Davis M, Ripamonti C, Bruera E, Davies A, Molassiotis A. 2016 updated MASCC/ESMO consensus recommendations: management of nausea and vomiting in advanced cancer. Supportive Care in Cancer 2016; 25: 333 -40.

Other references v Noble SIR, Murtagh FEM, Bausewein C, Johnson MJ. Reply to: MASCC/ESMO consensus recommendations for the management of nausea and vomiting in advanced cancer. Support Care Cancer 2017; 25: 2989 -90. v Ripamonti CI, Davies A, Bruera E, Molassiotis A, Walsh D. Response to letter to the editor referencing 2016 updated MASCC / ESMO consensus recommendations: management of nausea and vomiting in advanced cancer. Supportive Care in Cancer 2017; 25: 2991 -2.

Guideline history v Date of final search of literature: February 2016 v Date on-line publication: August 2016 v Date of review of guideline: August 2021

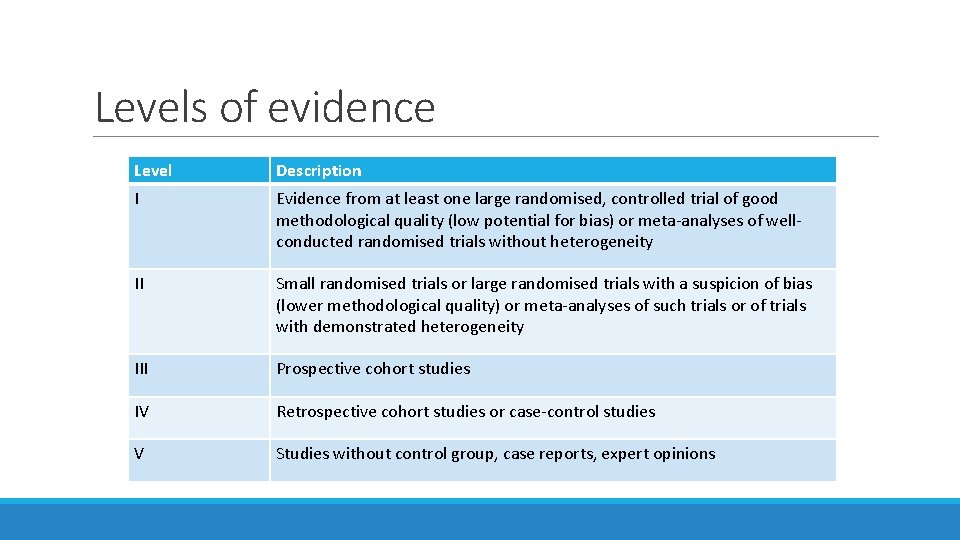
Levels of evidence Level Description I Evidence from at least one large randomised, controlled trial of good methodological quality (low potential for bias) or meta-analyses of wellconducted randomised trials without heterogeneity II Small randomised trials or large randomised trials with a suspicion of bias (lower methodological quality) or meta-analyses of such trials or of trials with demonstrated heterogeneity III Prospective cohort studies IV Retrospective cohort studies or case-control studies V Studies without control group, case reports, expert opinions

Grades of recommendation Grade Description A Strong evidence for efficacy with a substantial clinical benefit, strongly recommended B Strong or moderate evidence for efficacy but with a limited clinical benefit, generally recommended C Insufficient evidence for efficacy or benefit does not outweigh the risk or the disadvantages (adverse events, costs, …), optional D Moderate evidence against efficacy or for adverse outcome, generally not recommended E Strong evidence against efficacy or for adverse outcome, never recommended

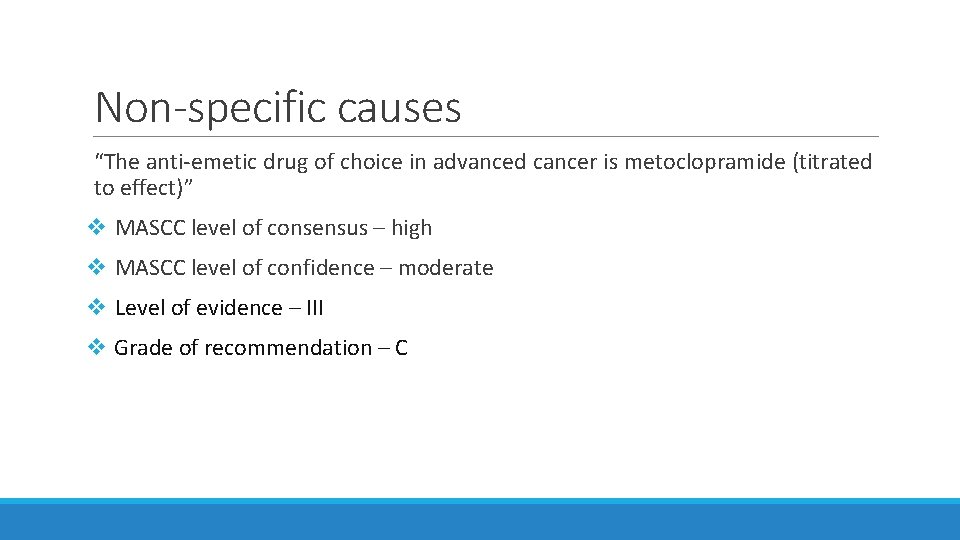
Non-specific causes “The anti-emetic drug of choice in advanced cancer is metoclopramide (titrated to effect)” v MASCC level of consensus – high v MASCC level of confidence – moderate v Level of evidence – III v Grade of recommendation – C

Non-specific causes “Alternative options include haloperidol, levomepromazine, or olanzapine” v MASCC level of consensus – high v MASCC level of confidence – low v Level of evidence – V v Grade of recommendation – D

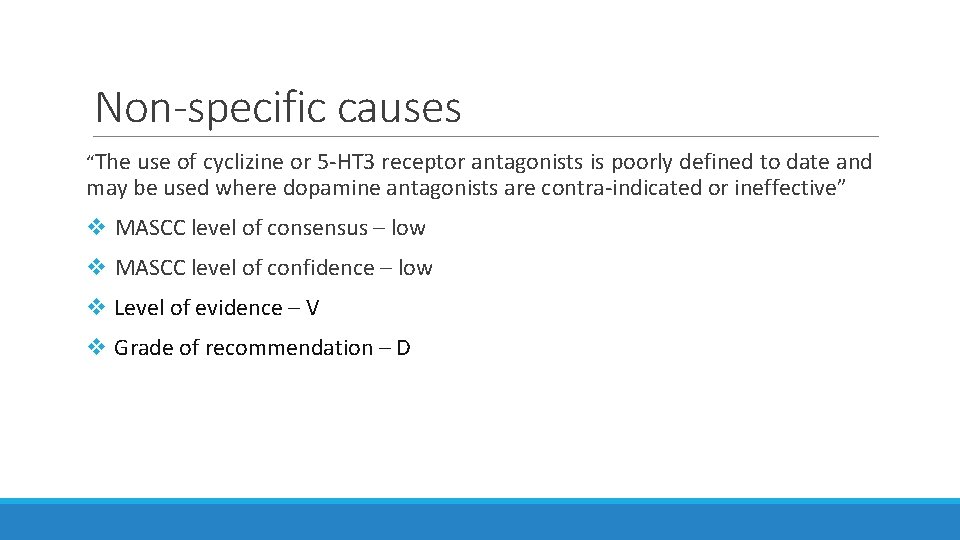
Non-specific causes “The use of cyclizine or 5 -HT 3 receptor antagonists is poorly defined to date and may be used where dopamine antagonists are contra-indicated or ineffective” v MASCC level of consensus – low v MASCC level of confidence – low v Level of evidence – V v Grade of recommendation – D

Bowel obstruction “The drug recommended in a bowel obstruction is octreotide, dosed around the clock, and given alongside an antiemetic (with the committee recommending haloperidol)” v MASCC level of consensus – high v MASCC level of confidence – high v Level of evidence – II v Grade of recommendation – A

Bowel obstruction “If octreotide plus antiemetic is ineffective, the use of anti-cholinergic antisecretory agents (e. g. , scopolamine butylbromide, glycopyrronium bromide) and/or corticosteroids is recommended as either adjunct or alternative interventions” v MASCC level of consensus – high (moderate for corticosteroids) v MASCC level of confidence – moderate (low for corticosteroids) v Level of evidence – IV v Grade of recommendation – D

Bowel obstruction “The use of cyclizine or 5 -HT 3 receptor antagonists is poorly defined in this setting. Metoclopramide should be used with caution in partial bowel obstruction and should not be used in complete bowel obstruction” v MASCC level of consensus – low v MASCC level of confidence – low v Level of evidence – V v Grade of recommendation – D

Opioid-induced “No recommendation can be made for specific antiemetics, although various antiemetics may help. Opioid rotation and route switching may be effective approaches. There is no data to support prophylactic antiemetics in this situation” v MASCC level of consensus – high v MASCC level of confidence – low v Level of evidence – V v Grade of recommendation – D