Marks 10 halfmed exam 10 final exam 2










- Slides: 33


Marks • 10 half-med exam • 10 final exam • 2 experiments + lab cleaning • 3 attending


Molecular Biology is a field of science aims to understand the basis of living organisms’ chemical reactions necessary to build cell’s nutrients and to perform biological functions

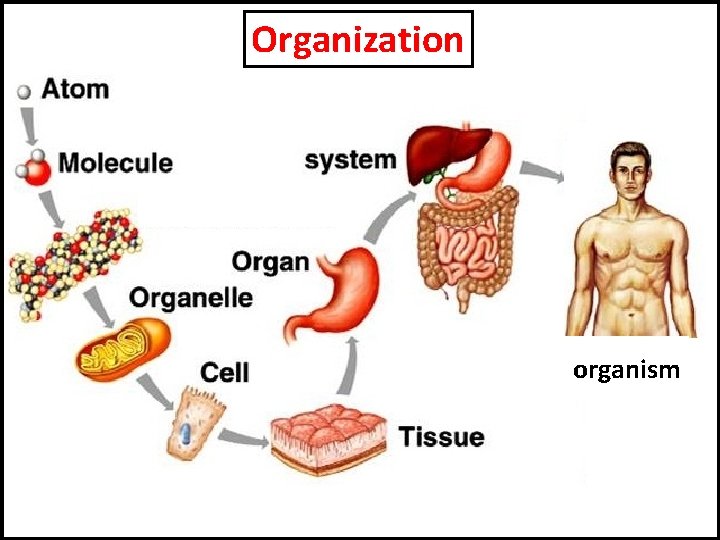
Organization organism

Types of Molecules • Inorganic molecules • Organic molecules

Macromolecules • Proteins • Carbohydrates • Lipids • Nucleic acids

Proteins in cells & Tissues Proteins: One of the 4 macromolecules in living organisms, they are composed by binding of amino acids to each other with peptide bonds

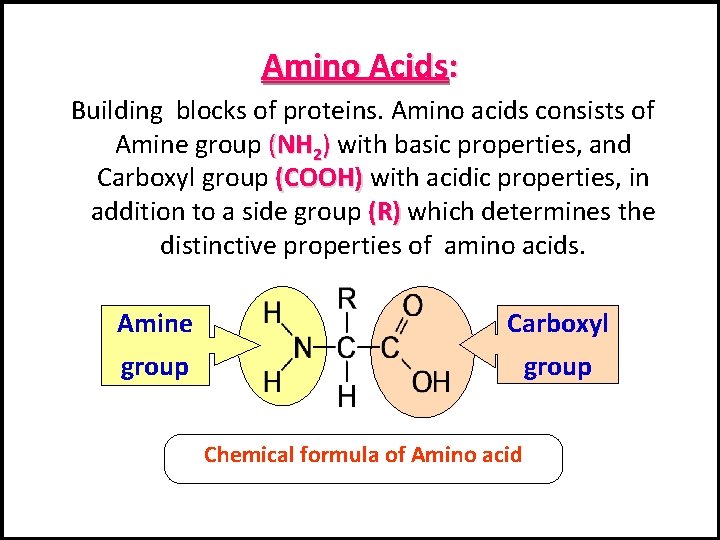
Amino Acids: Building blocks of proteins. Amino acids consists of Amine group (NH 2) with basic properties, and Carboxyl group (COOH) with acidic properties, in addition to a side group (R) which determines the distinctive properties of amino acids. Amine Carboxyl group Chemical formula of Amino acid


Amino acids are divided into many kinds dependents to R group: -Physical properties -Chemical properties -Biological properties

• Neutral non polar amino acids: e. g. Tryptophan • Neutral polar amino acids: e. g. Cysteine. • Non-neutral amino acids: e. g. Arginine.

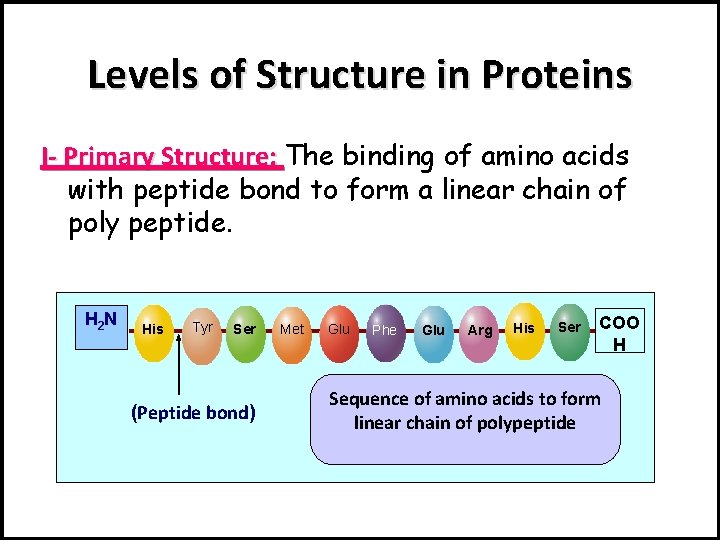
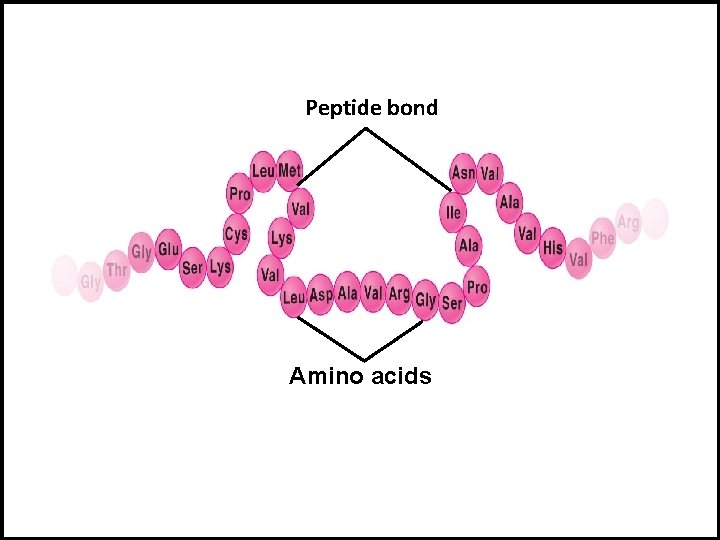
Levels of Structure in Proteins I- Primary Structure: The binding of amino acids with peptide bond to form a linear chain of poly peptide. H 2 N His Tyr Ser (Peptide bond) Met Glu Phe Glu Arg His Ser COO H Sequence of amino acids to form linear chain of polypeptide

Peptide bond Amino acids

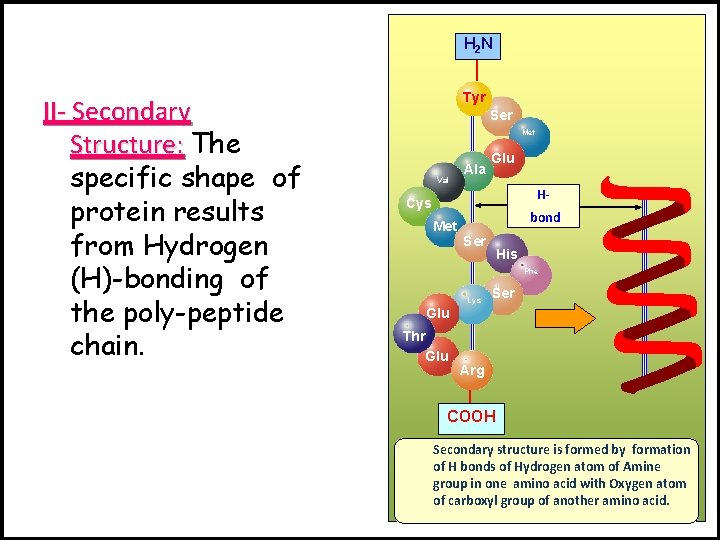
H 2 N II- Secondary Structure: The specific shape of protein results from Hydrogen (H)-bonding of the poly-peptide chain. Tyr Ser Met Val Ala Glu H- Cys Met bond Ser His Phe Lys Ser Glu Thr Glu Arg COOH Secondary structure is formed by formation of H bonds of Hydrogen atom of Amine group in one amino acid with Oxygen atom of carboxyl group of another amino acid.

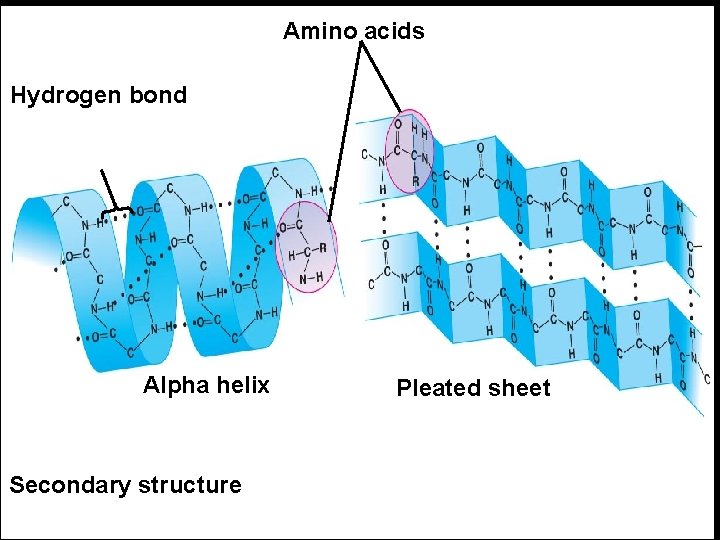
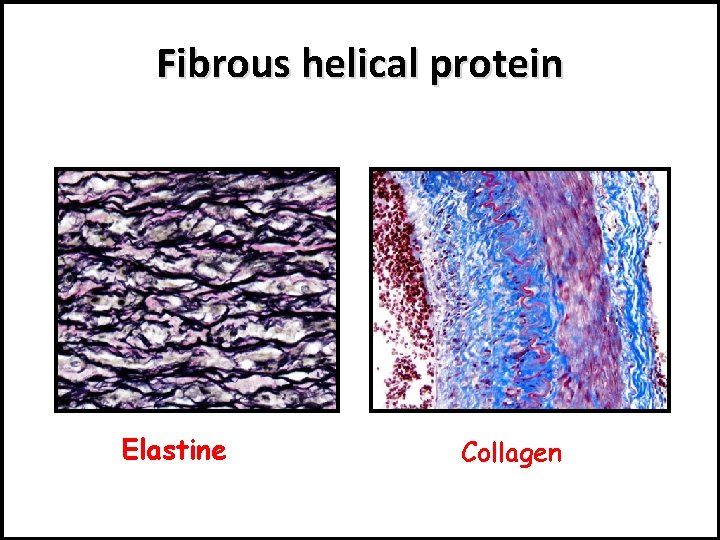
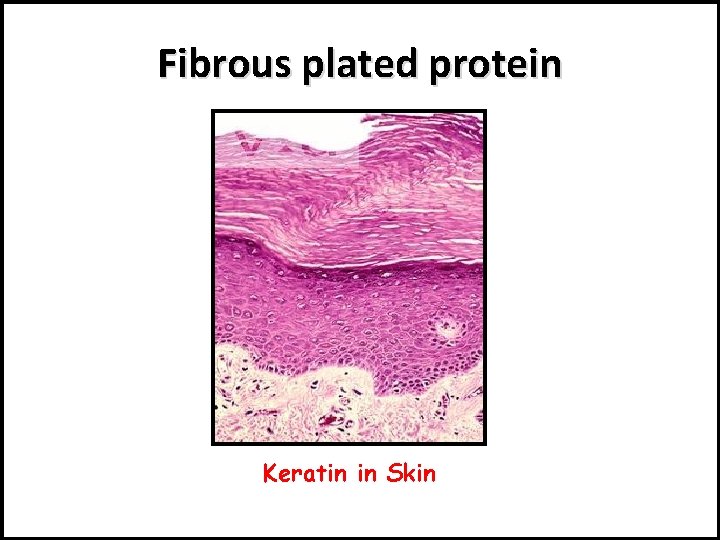
There are 2 forms of secondary structure: 1 - α-helix : e. g. Collagen protein in white fibers, and Elastine in elastic fibers. 2 - β- plated sheet: e. g. Keratin protein in hair and horny layer of skin.

Amino acids Hydrogen bond Alpha helix Secondary structure Pleated sheet

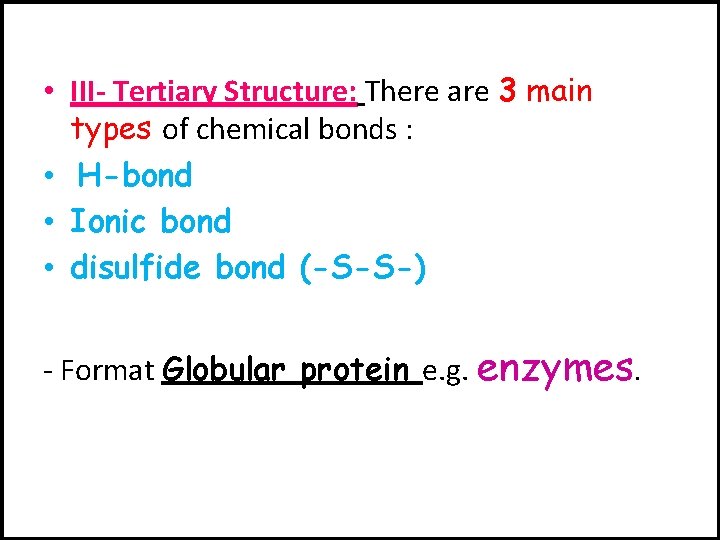
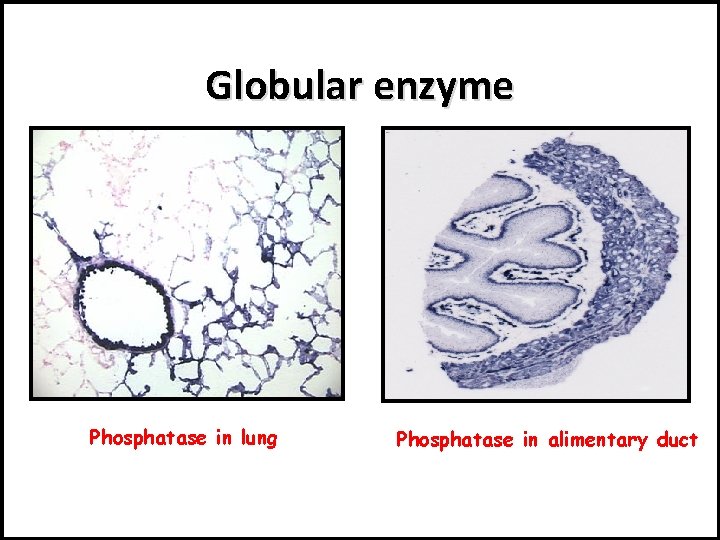
• III- Tertiary Structure: There are 3 main types of chemical bonds : • H-bond • Ionic bond • disulfide bond (-S-S-) - Format Globular protein e. g. enzymes.


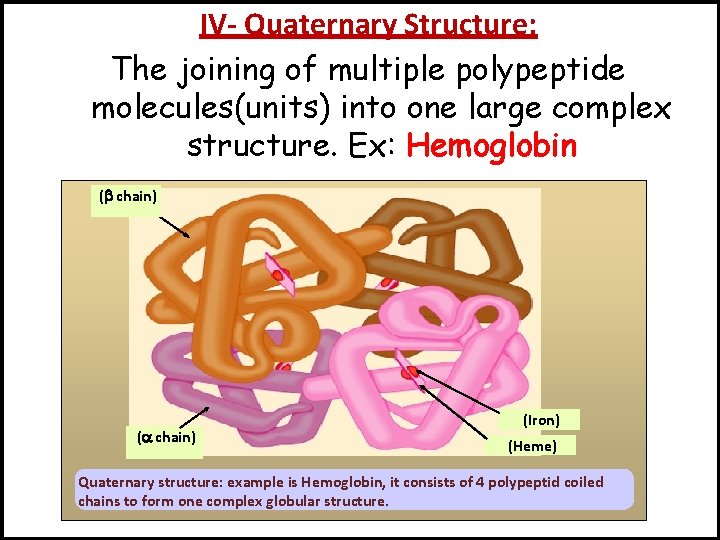
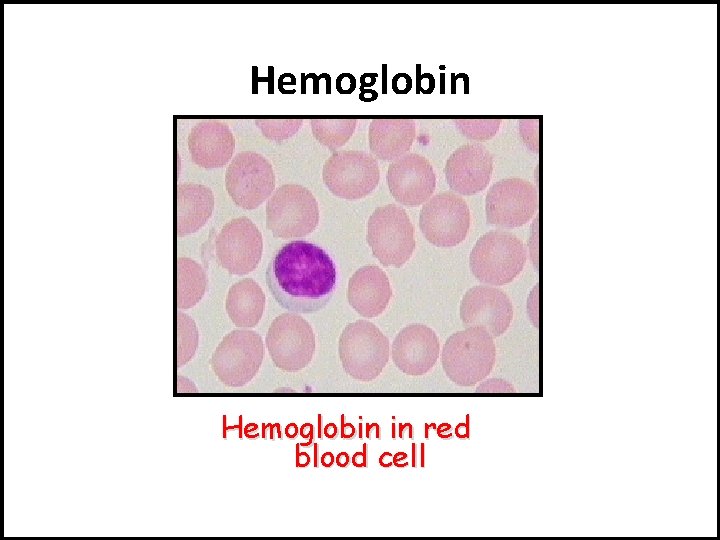
IV- Quaternary Structure: The joining of multiple polypeptide molecules(units) into one large complex structure. Ex: Hemoglobin ( chain) (Iron) (Heme) Quaternary structure: example is Hemoglobin, it consists of 4 polypeptid coiled chains to form one complex globular structure.


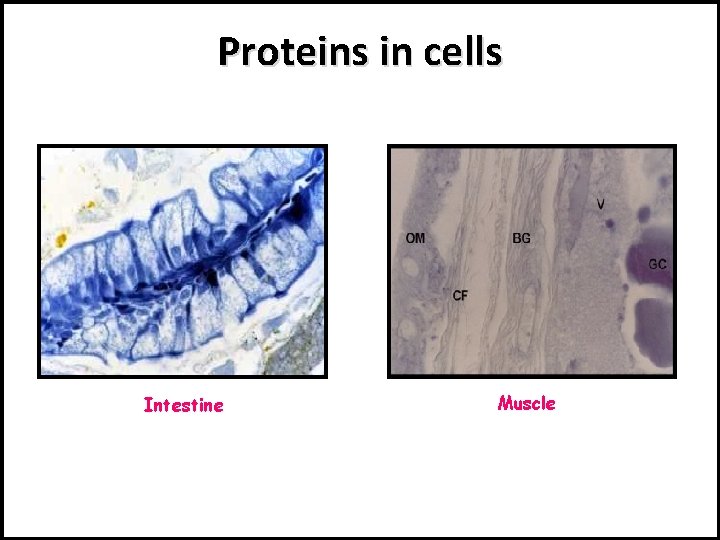
Proteins in cells Intestine Muscle

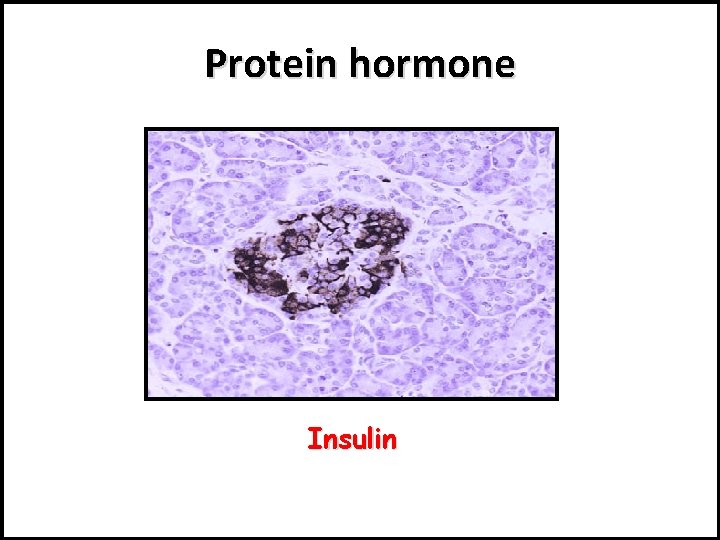
Protein hormone Insulin

Fibrous helical protein Elastine Collagen

Fibrous plated protein Keratin in Skin

Globular enzyme Phosphatase in lung Phosphatase in alimentary duct

Hemoglobin in red blood cell

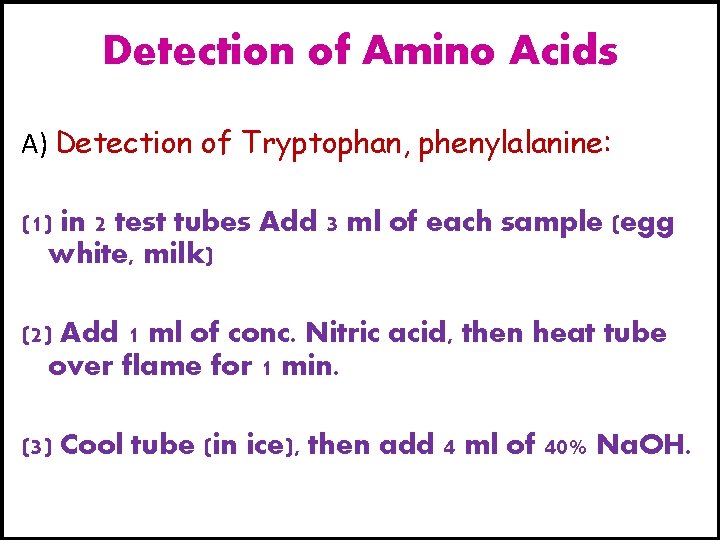
Detection of Amino Acids A) Detection of Tryptophan, phenylalanine: (1) in 2 test tubes Add 3 ml of each sample (egg white, milk) (2) Add 1 ml of conc. Nitric acid, then heat tube over flame for 1 min. (3) Cool tube (in ice), then add 4 ml of 40% Na. OH.


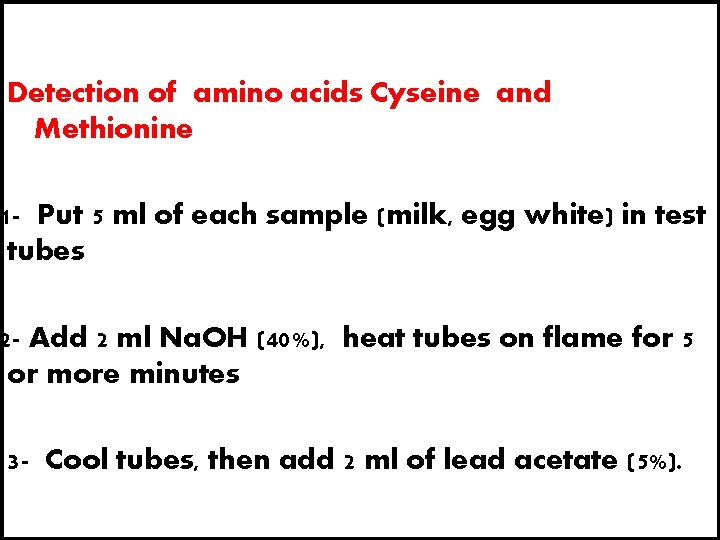
Detection of amino acids Cyseine and Methionine 1 - Put 5 ml of each sample (milk, egg white) in test tubes 2 - Add 2 ml Na. OH (40%), heat tubes on flame for 5 or more minutes 3 - Cool tubes, then add 2 ml of lead acetate (5%).


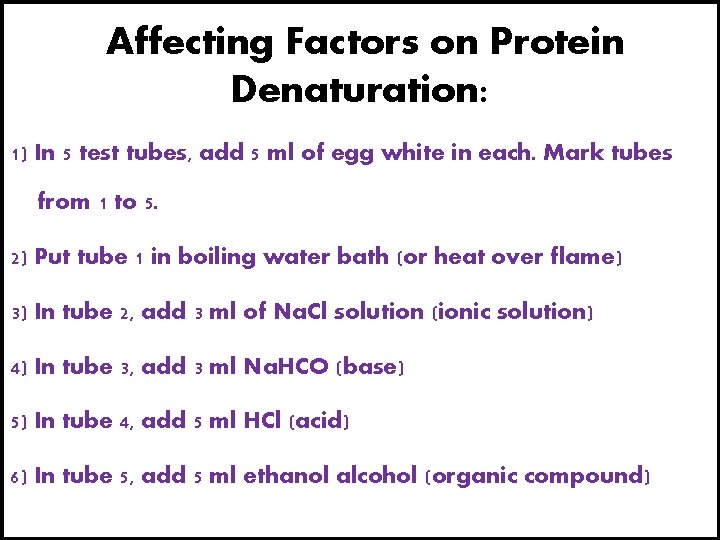
Affecting Factors on Protein Denaturation: 1) In 5 test tubes, add 5 ml of egg white in each. Mark tubes from 1 to 5. 2) Put tube 1 in boiling water bath (or heat over flame) 3) In tube 2, add 3 ml of Na. Cl solution (ionic solution) 4) In tube 3, add 3 ml Na. HCO (base) 5) In tube 4, add 5 ml HCl (acid) 6) In tube 5, add 5 ml ethanol alcohol (organic compound)

Thank you for listening