Many familiar compounds are acids or bases Lemon


• Many familiar compounds are acids or bases. – Lemon juice, soap, oranges, pop… • Acids and bases can be very dangerous. – Both can be very corrosive. • NEVER try to identify an acid or base by taste or touch! See pages 220 - 222 (c) Mc. Graw Hill Ryerson 2007

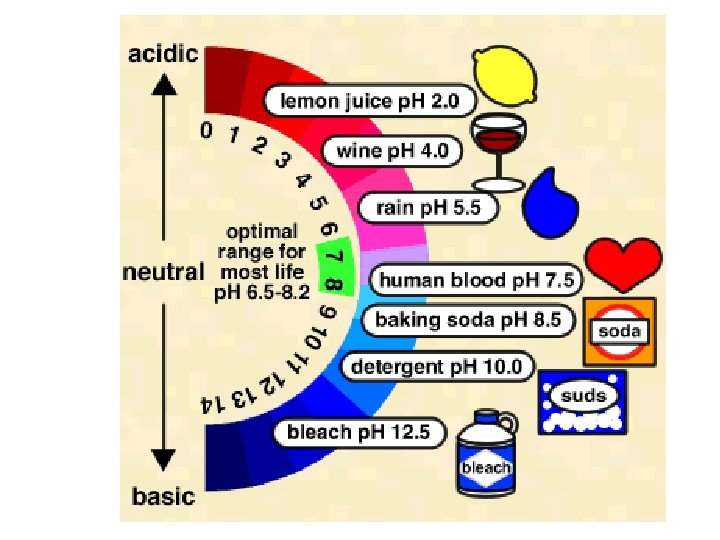
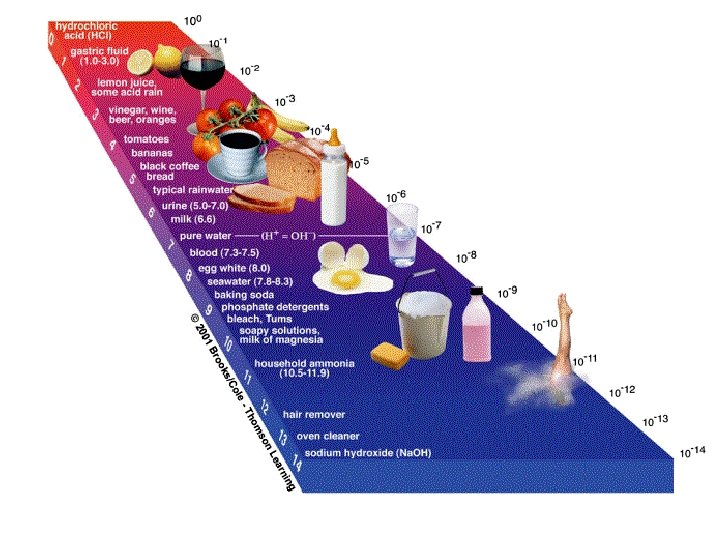
• The strength of acids and bases is measured on the p. H scale. – p. H below 7 = acidic – p. H above 7 = basic – p. H 7 = neutral – 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 Acids Neutral Bases See pages 220 - 222 (c) Mc. Graw Hill Ryerson 2007
![[ H +] Acids release _____ into a solution. [OH-] Bases release _____ into [ H +] Acids release _____ into a solution. [OH-] Bases release _____ into](http://slidetodoc.com/presentation_image_h2/1871b144cb7d6e0354a10a240562a056/image-4.jpg)
[ H +] Acids release _____ into a solution. [OH-] Bases release _____ into a solution.
![+] [ H p. H is the concentration of ____ expressed as a logarithm. +] [ H p. H is the concentration of ____ expressed as a logarithm.](http://slidetodoc.com/presentation_image_h2/1871b144cb7d6e0354a10a240562a056/image-5.jpg)
+] [ H p. H is the concentration of ____ expressed as a logarithm. Every change in the p. H scale of one unit is a change in 10 times the concentration of H+. Example: ______ • p. H 4 is 10 X more acidic than p. H 5. • p. H 3 is 1000 X more acidic than p. H 5.
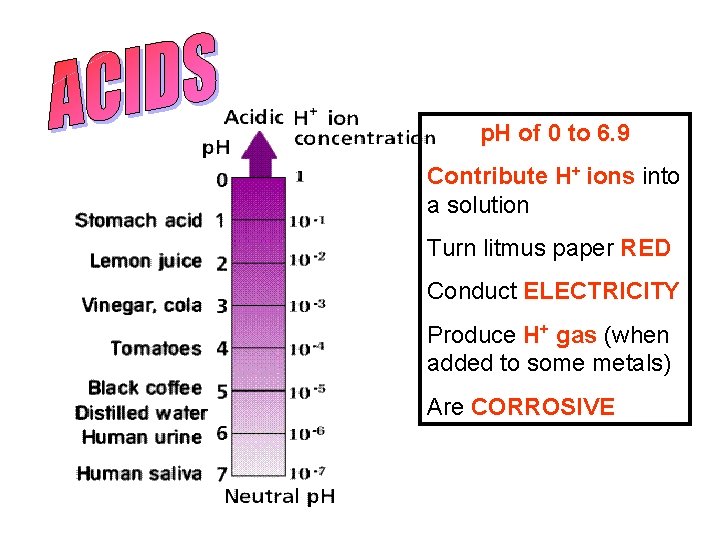
p. H of 0 to 6. 9 Contribute H+ ions into a solution Turn litmus paper RED Conduct ELECTRICITY Produce H+ gas (when added to some metals) Are CORROSIVE
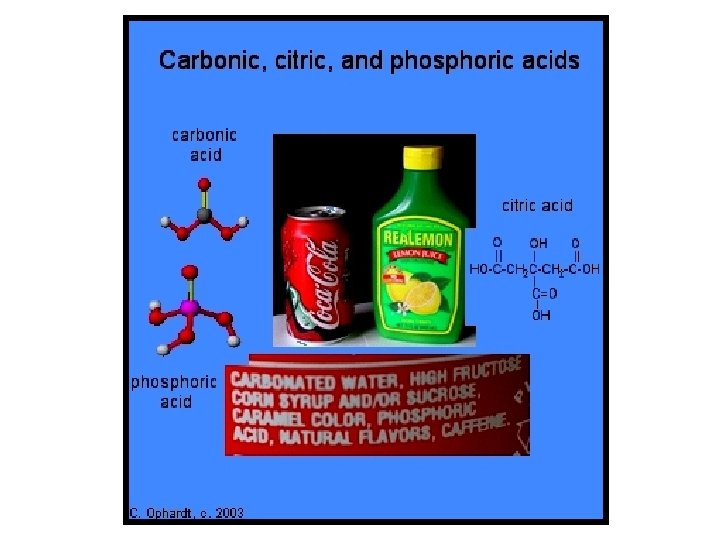
• Acids often behave like acids only when dissolved in water. • Therefore, acids often are written with symbol (aq) = aqueous = water. • The chemical formula of an acid usually starts with hydrogen (H). –HCl(aq) = hydrochloric acid, HNO 3(aq) = nitric acid • Acids with a carbon usually have the C written first. • CH 3 COOH(aq) = acetic acid See pages 225 - 226 (c) Mc. Graw Hill Ryerson 2007

Sulfuric acid is used in batteries. • Naming acids: If you know a compound’s chemical formula, you may be able to identify whether it as an acid. – Hydrogen + …-ide = hydro…ic acid • HF(aq) = hydrogen fluoride = hydrofluoric acid – Hydrogen + …-ate = …ic acid • H 2 CO 3(aq) = hydrogen carbonate = carbonic acid – Hydrogen + …-ite = …ous acid • H 2 SO 3(aq) = hydrogen sulphite = sulphurous acid See pages 225 - 226 (c) Mc. Graw Hill Ryerson 2007

![p. H of 7. 1 to 14 Donate [OH]- ions into a solution The p. H of 7. 1 to 14 Donate [OH]- ions into a solution The](http://slidetodoc.com/presentation_image_h2/1871b144cb7d6e0354a10a240562a056/image-13.jpg)
p. H of 7. 1 to 14 Donate [OH]- ions into a solution The hydroxide [OH-] group is neutralized by a hydrogen ion and water is formed. Turns litmus paper BLUE Conduct ELECTRICITY Are Slippery Are CAUSTIC

• Bases often behave like bases only when dissolved in water. • Therefore, bases often are written with symbol (aq) = aqueous = water. • If you know a compound’s chemical formula, you may be able to identify it as a base. • The chemical formula of a base usually ends with hydroxide (OH). (c) Mc. Graw Hill Ryerson 2007

• Bases can be gentle or very caustic. • Examples of common bases: – Na. OH(aq) – Mg(OH)2(aq) – Ca(OH)2(aq) – NH 4 OH(aq) See page 227 (c) Mc. Graw Hill Ryerson 2007

The p. H of almost all living systems is between p. H 6 -8 ____. Maintaining the proper p. H then is vital living systems. p. H is very important to the environment as well: • acid rain • agriculture

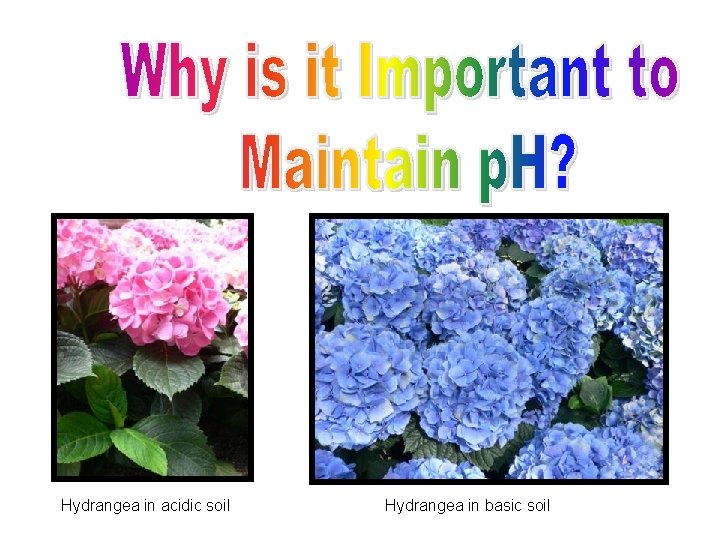
Hydrangea in acidic soil Hydrangea in basic soil
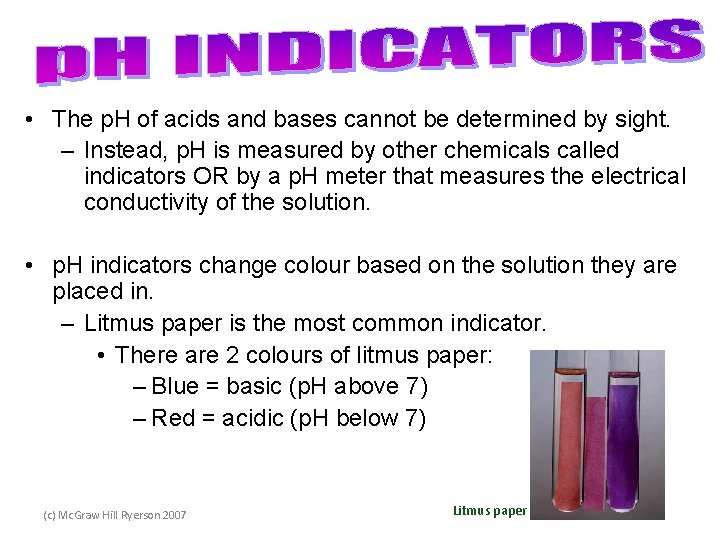
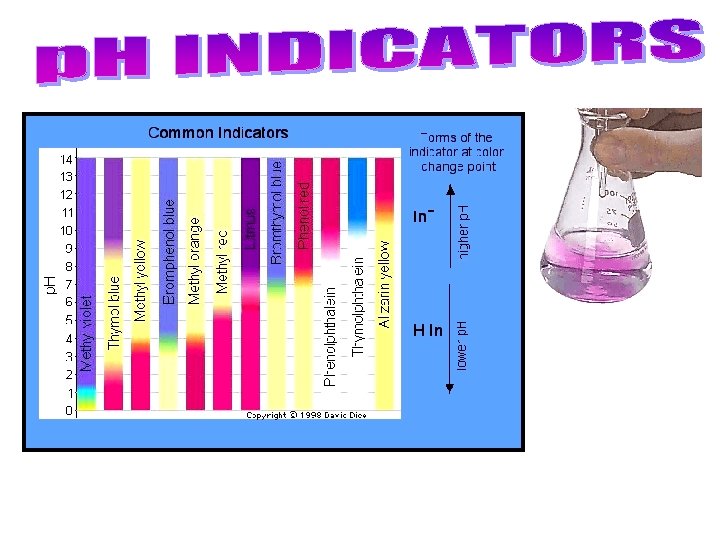
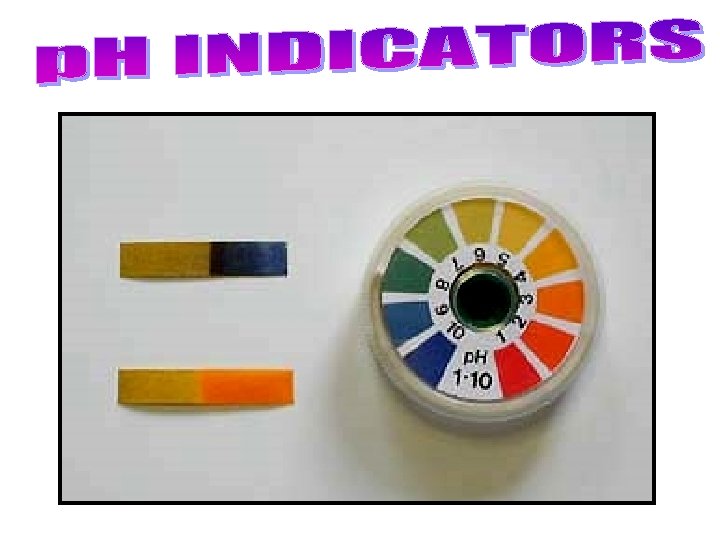
• The p. H of acids and bases cannot be determined by sight. – Instead, p. H is measured by other chemicals called indicators OR by a p. H meter that measures the electrical conductivity of the solution. • p. H indicators change colour based on the solution they are placed in. – Litmus paper is the most common indicator. • There are 2 colours of litmus paper: – Blue = basic (p. H above 7) – Red = acidic (p. H below 7) (c) Mc. Graw Hill Ryerson 2007 Litmus paper
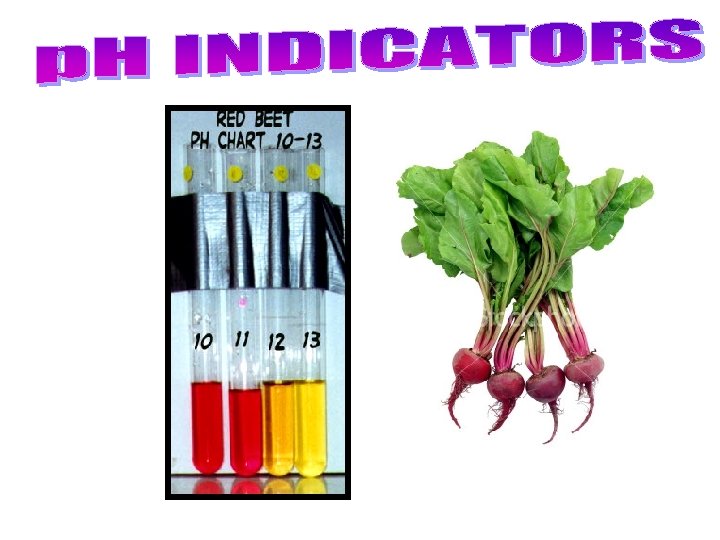
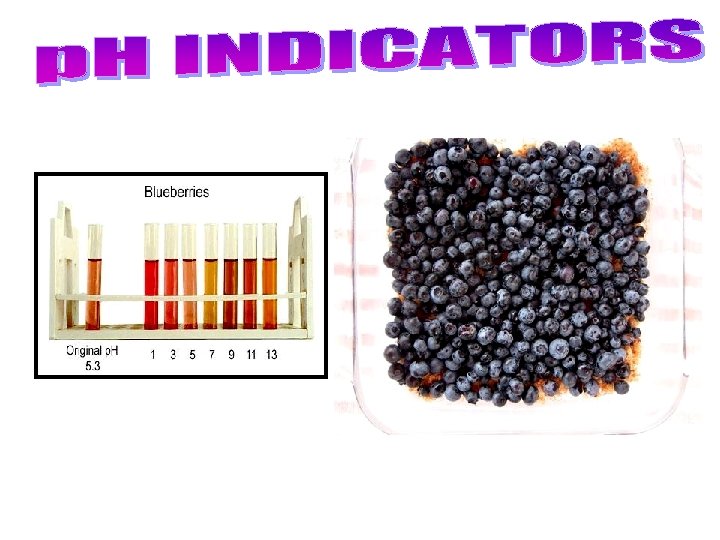
– A universal indicator contains many indicators that turn different colours at different p. H values • can be in liquid form, or on paper strips like litmus. – Indicators change colour at different p. H values, so different indicators are used to identify different p. H values. • Bromothymol blue for p. H 6 – 7. 6, phenolphthalein for p. H 8. 2 – 10. • Many natural sources, such as beets, blueberries, cabbage, pansy flower petals. . . are also indicators. See pages 223 - 224 (c) Mc. Graw Hill Ryerson 2007
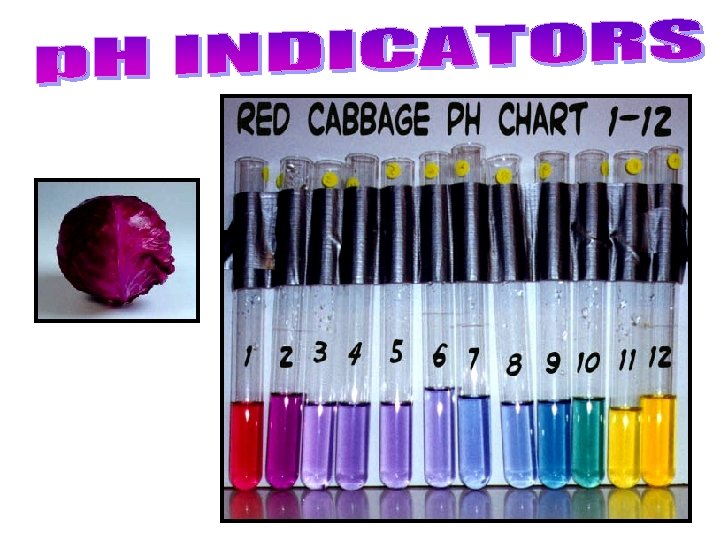


Cabbage Juice paper in baking soda (BASE) Cabbage Juice paper in lemon juice (ACID)
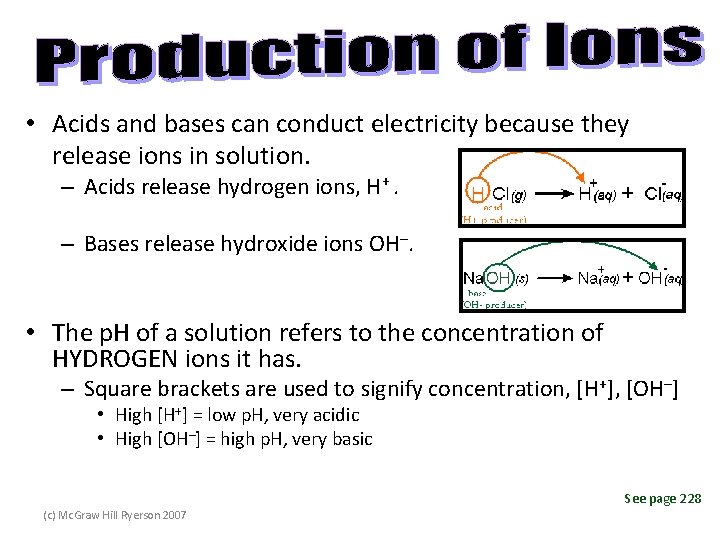
• Acids and bases can conduct electricity because they release ions in solution. – Acids release hydrogen ions, H+. – Bases release hydroxide ions OH–. • The p. H of a solution refers to the concentration of HYDROGEN ions it has. – Square brackets are used to signify concentration, [H+], [OH–] • High [H+] = low p. H, very acidic • High [OH–] = high p. H, very basic See page 228 (c) Mc. Graw Hill Ryerson 2007
![– A solution cannot have BOTH high [H+] and [OH–]; they cancel each other – A solution cannot have BOTH high [H+] and [OH–]; they cancel each other](http://slidetodoc.com/presentation_image_h2/1871b144cb7d6e0354a10a240562a056/image-29.jpg)
– A solution cannot have BOTH high [H+] and [OH–]; they cancel each other out and form water. – This process is called neutralization. – H+ + OH– H 2 O OR H+ + OH– HOH See page 228 NEUTRALIZATION: http: //www. youtube. com/watch? v=_P 5 h. Gz. A 6 Vb 0&feature=related
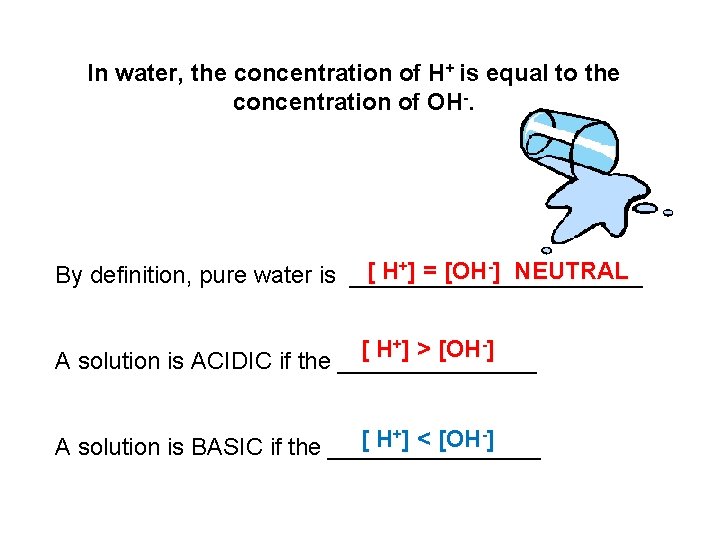
In water, the concentration of H+ is equal to the concentration of OH-. [ H+] = [OH-] NEUTRAL By definition, pure water is ___________ [ H+] > [OH-] A solution is ACIDIC if the ________ +] < [OH-] [ H A solution is BASIC if the ________
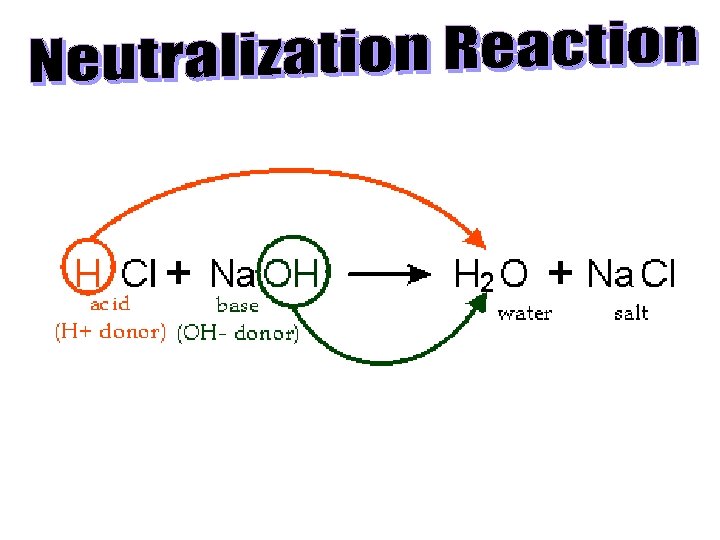
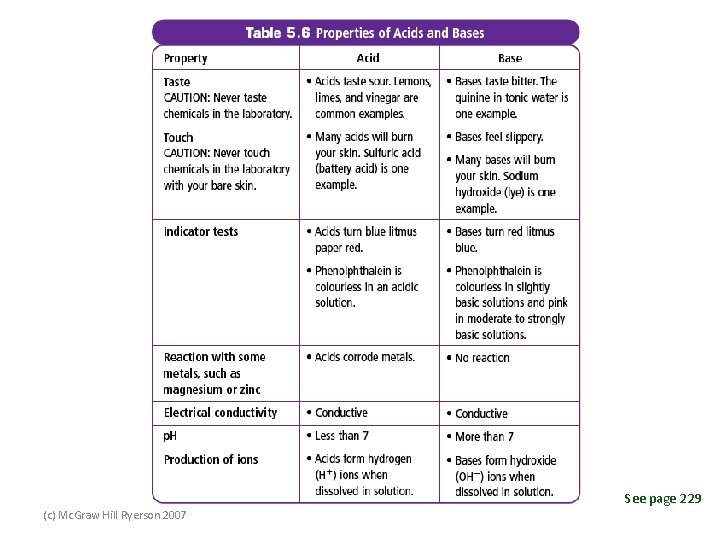
See page 229 (c) Mc. Graw Hill Ryerson 2007
- Slides: 32