Managing Clinical Pharmacy Services John M Allen Pharm



























- Slides: 41

Managing Clinical Pharmacy Services John M. Allen, Pharm. D, BCPS, CPh Pharmacy Clinical Coordinator Medical Center of Trinity

Lesson Objectives Differentiate between clinical and operational aspects of institutional pharmacy Describe the purpose of a hospital formulary Discuss principles of formulary management Describe the drug use evaluation process Identify different types of pharmacy practice models Describe the process for implementing new clinical services

What are Clinical Pharmacy Services? Activities and processes that: Ensure optimal therapeutic outcomes Minimize adverse drug events (ADEs) Promote cost-effective strategies Can be provided in all health-settings Not limited to institutional settings

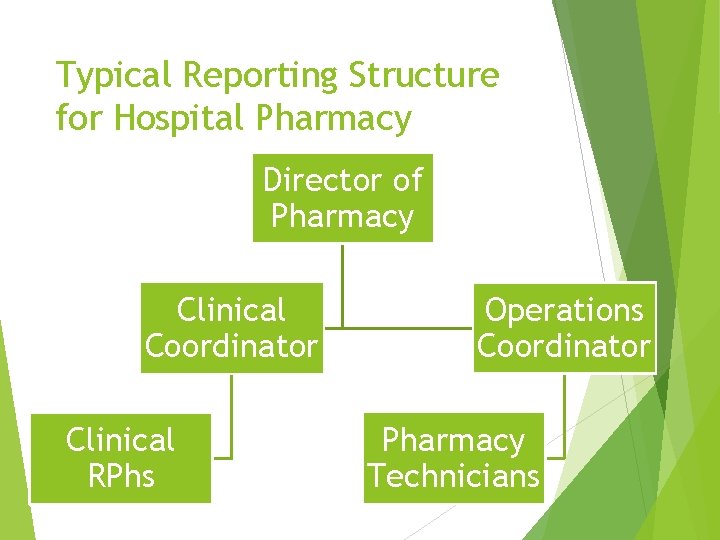
Typical Reporting Structure for Hospital Pharmacy Director of Pharmacy Clinical Coordinator Clinical RPhs Operations Coordinator Pharmacy Technicians

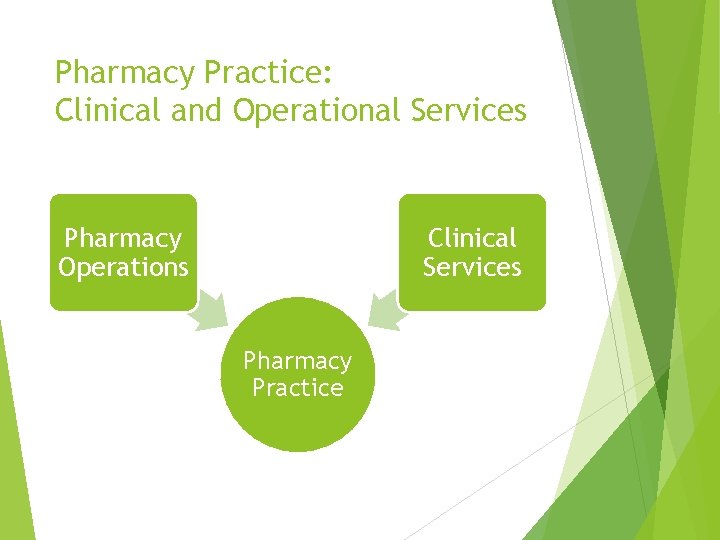
Pharmacy Practice: Clinical and Operational Services Pharmacy Operations Clinical Services Pharmacy Practice

Case Dr. Smith, chief of cardiology at your institution has requested that a new cholesterol drug De-Clogo be added to hospital formulary The drug was recently approved by the FDA and has a similar mechanism to other formulary alternatives You as the Clinical Coordinator are asked to review and present the drug at your hospital’s next Pharmacy and Therapeutics (P&T) Committee meeting What things should be considered when evaluating addition of a new drug to formulary?

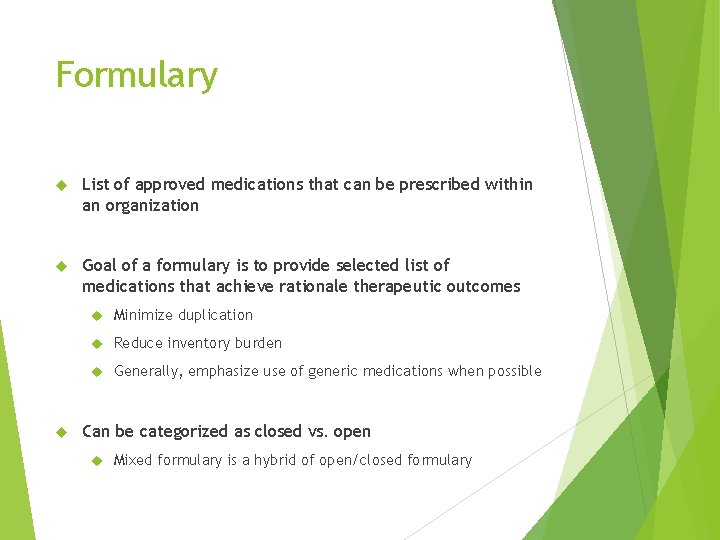
Formulary List of approved medications that can be prescribed within an organization Goal of a formulary is to provide selected list of medications that achieve rationale therapeutic outcomes Minimize duplication Reduce inventory burden Generally, emphasize use of generic medications when possible Can be categorized as closed vs. open Mixed formulary is a hybrid of open/closed formulary

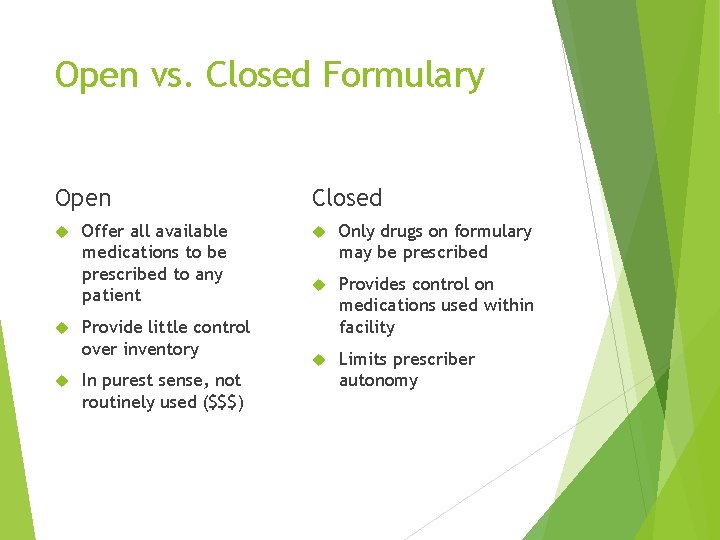
Open vs. Closed Formulary Open Offer all available medications to be prescribed to any patient Provide little control over inventory In purest sense, not routinely used ($$$) Closed Only drugs on formulary may be prescribed Provides control on medications used within facility Limits prescriber autonomy

Mixed Formulary System Most common type of formulary system used by hospitals and hospital-systems Allows flexibility within formulary Generally, within mixed formulary lies three tiers Open- Any drug on formulary may be prescribed for any patient Restricted- Medication is limited to specific patient population or prescribers (i. e. Chemotherapy) Targeted- Medication requires approval prior to dispensing (i. e. Targeted antimicrobials ID physician or Pharm. D )

Formulary Management When considering adding new agents to formulary, a drug monograph is typically presented Evidence-based review of medical literature Standards exist to guide monograph development Can be developed internally or from outside vendor Facts and Comparison

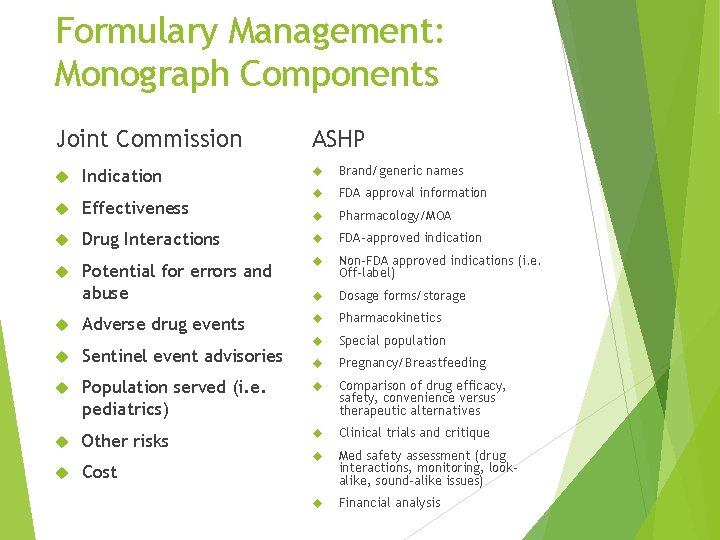
Formulary Management: Monograph Components Joint Commission Indication ASHP Brand/generic names FDA approval information Pharmacology/MOA Effectiveness Drug Interactions FDA-approved indication Potential for errors and abuse Non-FDA approved indications (i. e. Off-label) Dosage forms/storage Adverse drug events Pharmacokinetics Special population Pregnancy/Breastfeeding Sentinel event advisories Population served (i. e. pediatrics) Comparison of drug efficacy, safety, convenience versus therapeutic alternatives Other risks Clinical trials and critique Med safety assessment (drug interactions, monitoring, lookalike, sound-alike issues) Financial analysis Cost

Formulary Management Things to Consider Efficacy Safety Cost

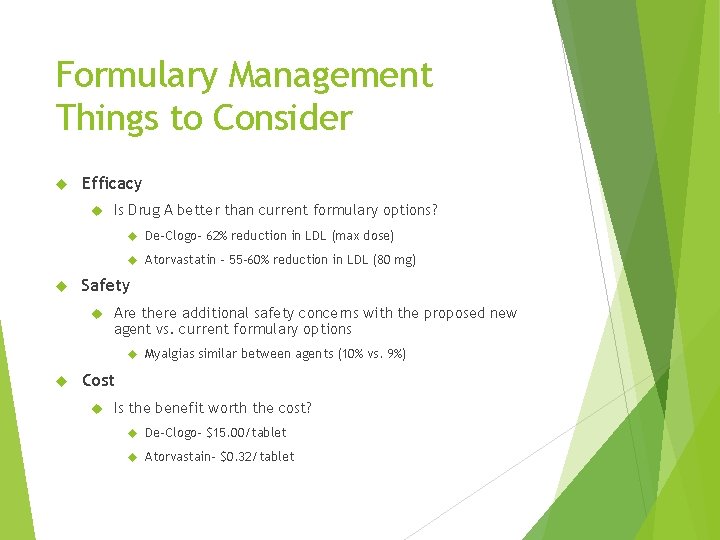
Formulary Management Things to Consider Efficacy Is Drug A better than current formulary options? De-Clogo- 62% reduction in LDL (max dose) Atorvastatin – 55 -60% reduction in LDL (80 mg) Safety Are there additional safety concerns with the proposed new agent vs. current formulary options Myalgias similar between agents (10% vs. 9%) Cost Is the benefit worth the cost? De-Clogo- $15. 00/tablet Atorvastain- $0. 32/tablet

Formulary Management Recommendations Generally, after review of drug monograph, formulary recommendations made Formulary Decisions Addition without restrictions Addition with restrictions on prescribers, or patient types (i. e. Criteria for use) Addition with required approval prior to dispensing Reject medication formulary addition 6 -to-12 month review for most new medications added to formulary to ensure appropriate use

Formulary System Policies and procedures which govern medication use process Policy- Course or plan of action Procedure- Course of action intended to achieve a result; provides details processes Medication use process Drug selection and procurement Ordering and transcribing Preparing and dispensing Administration Monitoring Should be developed in collaboration with multidisciplinary team (i. e. RN, MD, RT, etc. ) and evidence-based Policies and procedures to be reviewed periodically

Pharmacy & Therapeutics (P&T) Committee that oversees safe, effective, and responsible medication use within hospital Comprised of physicians, nurses, pharmacists, hospital administrators, support staff, Hospital Quality Medical committee NOT Pharmacy Committee Conflict of interest should be disclosed annually Stock investments, Research support, Speakers Bureau, etc. Recommendations are subject to Medical Executive Committee Approval

P&T Committee Subcommittees Provide expert advise on the development and monitoring of processes for specific groups of drugs Antimicrobial subcommittee Anticoagulation committee Pain management committee Pediatrics committee Subcommittees typically complete drug use evaluations (DUEs) and develop evidence-based tools to guide appropriate drug therapy Criteria for Use Orderset development Education materials

Case The Director of Pharmacy has asked you to evaluate drug spend for the institution and look for opportunities to reduce overall drug spend You notice that Procrit (epoetin alfa) spend is increased significantly from previous year You suspect that Procrit utilization is increased due to inappropriate use and have decided to complete a DUE on Procrit What things should you consider when completing a DUE?

Drug Use Evaluation Term used synonymously with Medication Use Evaluation (MUE) Drug Use Evaluation required by The Joint Commission to monitor safety of medications Structured, quality improvement program designed to promote appropriate, safe, and effective medication use

Drug Use Evaluation Examples of Medications/High-Risk Medication Related Processes Neuromuscular blocking agents Anticoagulants Insulin infusion Sedation protocols Stress Ulcer Prophylaxis Albumin Epoetin Alfa Management of Clostridium Difficile Infection

Drug Use Evaluation Process Gain Organizational Authority and Assign Responsibility Identify areas of opportunity Develop criteria and indicators for optimal use Involve practitioners who practice in the setting Collect data and evaluate utilization Develop and implement plans for improvement Monitor and assess plan for improvement

Pharmacy Practice Models Describes how a pharmacy department's resources are deployed to provide patient care Includes pharmacists, technicians, automation, and technology Pharmacy Practice Model Initiative (PPMI), 2010 Sponsored by ASHP Key opinion leaders in pharmacy practice Conclusions centered on moving from pharmacy-centric to patient-centric focus

Pharmacy Practice Model Initiative: Recommendations The imperative that all patients should have a right to the care of a pharmacist The characteristics, requirements, and challenges of optimal pharmacy models Advancing the application of information technology in the medication-use process Advancing the use of pharmacy technicians Successful implementation of new pharmacy practice models

Clinical Services UHC 2010 All patients Specialized Services Med reconciliation Anticoagulation management Review of non-emergent orders prior to first dose Resuscitation teams Parenteral nutrition Develop individualized treatment plans IV to PO conversion Antimicrobial stewardship Daily monitoring of medication profiles Pharmacokinetic evaluation, dosing, monitoring Participation in patient care rounds Renal dosing adjustments Collaborative drug therapy management Patient education on preventing disease and improving health Educate patients on new medications Communicate discharge plan to outside caregivers

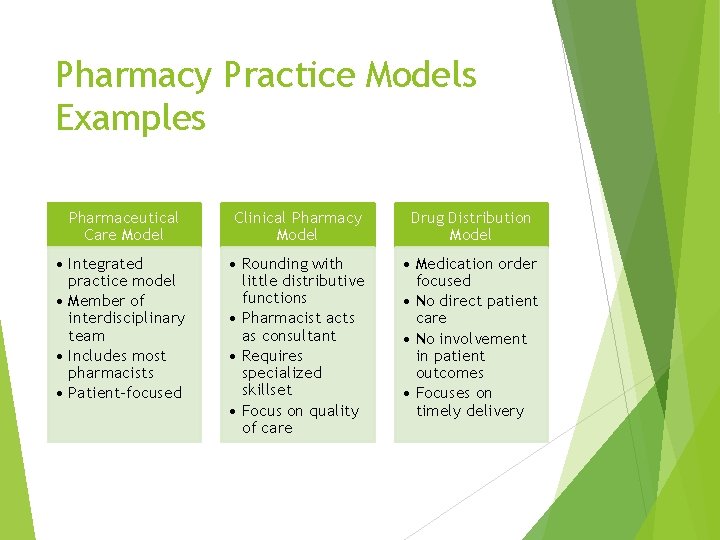
Pharmacy Practice Models Examples Pharmaceutical Care Model Clinical Pharmacy Model Drug Distribution Model • Integrated practice model • Member of interdisciplinary team • Includes most pharmacists • Patient-focused • Rounding with little distributive functions • Pharmacist acts as consultant • Requires specialized skillset • Focus on quality of care • Medication order focused • No direct patient care • No involvement in patient outcomes • Focuses on timely delivery

Pharmacy Practice Models Choosing a model Depends on host of factors Logistical issues Utilization/deployment of pharmacy technicians Automation/Technology Pharmacist training Scope of pharmacy practice Standardized model not yet developed Agreement in some aspects Patient-centered care avoids/reduces med errors Involvement in patient centered care improves collaboration with physicians

Specific Practice Model: Critical Care Pharmacy Fundamental Distributive Order entry/verification Desirable Optimal Assist providers with therapeutic decision making Coordinate or direct residency training Formal nutrition consults Respond to resuscitation events Develop new pharmacy programs Didactic lectures to healthcare professionals Perform clinical research Develop implement ICU policies/protocols Present case reports

Specific Activities: Critical Care Pharmacy Prospective evaluation of all drug therapy Contribute to P&T committee Evaluation of appropriateness of therapy Activities on costcontainment Drug dosing adjustments Monitoring and preventing DDI and ADEs Define goals of therapy Documentation of services Reporting of ADEs Nutritional assessment Provision of drug info Participation in educational and institutional activities Check compatibilities of IV medications Prevention and treatment of life-threatening infections Safe and optimal use of technology

Documentation of Services Documentation shows diversity, effectiveness, cost and outcome of activities Outcomes of documentation Establish additional clinical services Expand roles of existing services Assess new processes Provide data for quality assurance or research Accreditation purposes Promotional reasons Assessment of financial impact

Implementing New Services: Overview Four stages to developing plan for new clinical services Complete assessment of current clinical services Review of literature supporting clinical services Development of clinical services Implementation and assessment of new services

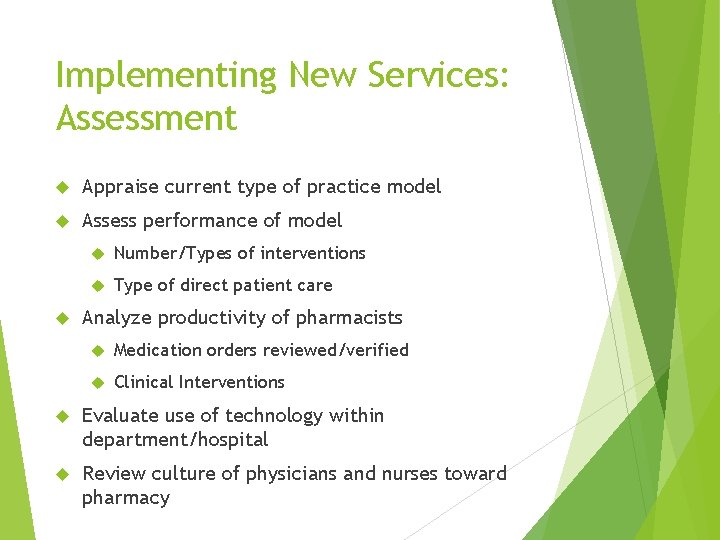
Implementing New Services: Assessment Appraise current type of practice model Assess performance of model Number/Types of interventions Type of direct patient care Analyze productivity of pharmacists Medication orders reviewed/verified Clinical Interventions Evaluate use of technology within department/hospital Review culture of physicians and nurses toward pharmacy

Implementing New Services: Review of current literature Summary of literature regarding clinical pharmacy service Impact of pharmacist intervention Identify and review pertinent health-system related data Patient outcome most associated with readmission rates Patient outcomes associated with reduction in payment from payors

Implementing New Services: Development Need clear vision and description of activities and expectation Mission statement Job description with clear expectations for clinical activities Metrics need to be developed to demonstrate effectiveness of clinical services Should be developed in concert with key stakeholders Example: Number/type of interventions, number of patients educated, number of med recs completed, drug cost avoided, time to verification

Implementing New Services: Implementation Most difficult phase Should include timeline for implementation Orientation and training of staff involved Electronic/logistical updates Development of infrastructure Ongoing monitoiring and feedback to pharmacists about clinical services Ongoing shadowing and coaching of clinical pharmacists

Managing Clinical Practitioners Required duty of pharmacy management Leadership and management skills Requires understanding of roles and responsibilities Self-reflection on state of clinical services Maintenance of relationships with key hospital personnel

Case Revisited Dr. Smith, chief of cardiology at your institution has requested that a new cholesterol drug De-Clogo be added to hospital formulary The drug was recently approved by the FDA and has a similar mechanism to other formulary alternatives You as the Clinical Coordinator are asked to review and present the drug at your hospital’s next Pharmacy and Therapeutics (P&T) Committee meeting What things should be considered when evaluating addition of a new drug to formulary?

Case Revisited When considering a new agent for potential formulary addition, drug monograph should be presented, and the following should be considered: Efficacy Safety issues Cost-effectiveness Based on review, De-Clogo should not be recommended formulary inclusion at this time Lack of significant benefit compared to current formulary options, and increased acquisition costs

Case Revisited The Director of Pharmacy has asked you to evaluate drug spend for the institution and look for opportunities to reduce overall drug spend You notice that Procrit (epoetin alfa) spend is increased significantly from previous year You suspect that Procrit utilization is increased due to inappropriate use and have decided to complete a DUE on Procrit What things should you consider when completing a DUE?

Case Revisited Review appropriate utilization of Epoetin alfa KDIGO Clinical Practice Guidelines Review appropriateness criteria with nephrologists Collect data and evaluate the information Develop opportunity for potential actions

Summary Formulary management is a key aspect to promoting safe, effective and responsible medication use within a hospital P&T Committee recommends formulary additions, formulary deletions, and processes designed to improve or guide medication use Drug Use Evaluations are a tool used to observe and identify opportunities to improve medication use Various practice models exist each with different areas of focus Implementation of new clinical services should be performed in a systematic way

Questions? Email: john. allen@hcahealthcare. com