MANAGEMENT OF PROTOCOL AND GCP DEVIATIONS AND VIOLATIONS





- Slides: 8

MANAGEMENT OF PROTOCOL AND GCP DEVIATIONS AND VIOLATIONS ACCORD SOP CR 010 v 5. 0 (Effective 01 October 2018) TRAINING SLIDES

DEFINITIONS • DEVIATION: Any change, divergence, or departure from the study design, procedures defined in the protocol or GCP that does not significantly affect a subjects rights, safety, or well-being, or study outcomes • VIOLATION: A deviation that may potentially significantly impact the completeness, accuracy, and/or reliability of the study data or that may significantly affect a subject’s rights, safety, or well-being

RESPONSIBILITIES • PI/research team responsibilities not changed • ACCORD QA; – Reviewing, logging, tracking and trending deviations/violations • ACCORD Senior Clinical Trials Monitor; – Awareness of study specific trends and/or significant findings – Discuss/address with Investigator

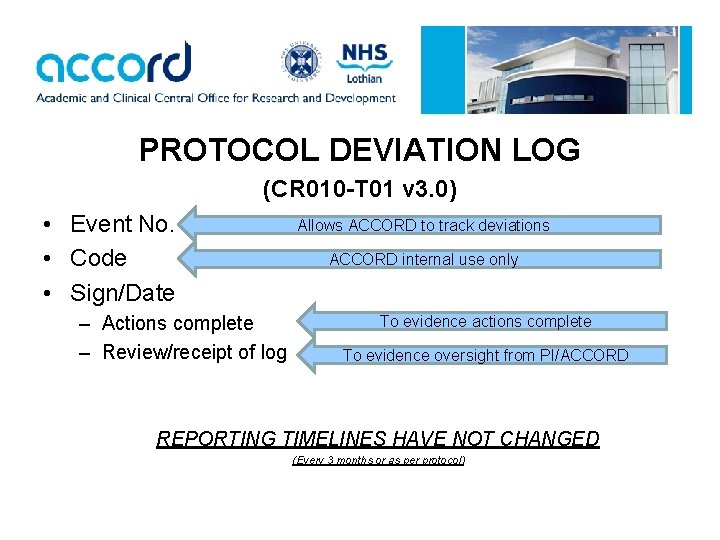
PROTOCOL DEVIATION LOG (CR 010 -T 01 v 3. 0) • Event No. • Code • Sign/Date – Actions complete – Review/receipt of log Allows ACCORD to track deviations ACCORD internal use only To evidence actions complete To evidence oversight from PI/ACCORD REPORTING TIMELINES HAVE NOT CHANGED (Every 3 months or as per protocol)

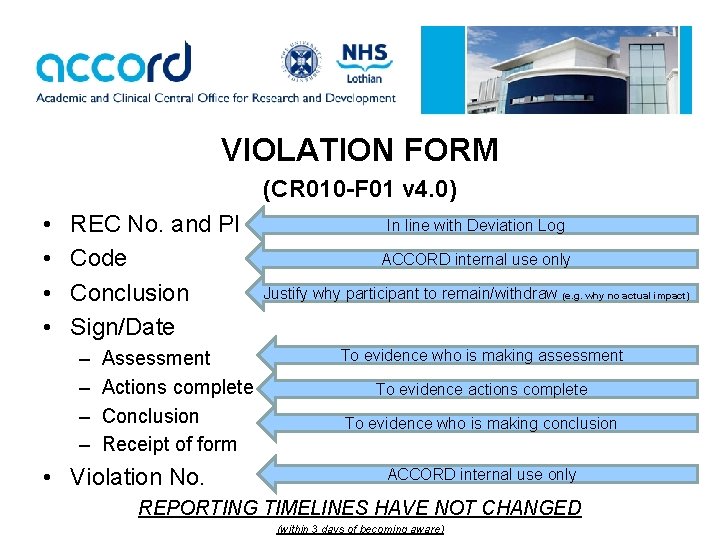
VIOLATION FORM (CR 010 -F 01 v 4. 0) • • REC No. and PI Code Conclusion Sign/Date – – Assessment Actions complete Conclusion Receipt of form • Violation No. In line with Deviation Log ACCORD internal use only Justify why participant to remain/withdraw (e. g. why no actual impact) To evidence who is making assessment To evidence actions complete To evidence who is making conclusion ACCORD internal use only REPORTING TIMELINES HAVE NOT CHANGED (within 3 days of becoming aware)

TRENDING • ACCORD QA will review/report on deviations/violations – Quarterly – Study trends – Global trends To Senior Management Team/Senior Clinical Trials Monitor (ACCORD) • ACCORD Senior Clinical Trials Monitor will address study specific trends and/or significant findings with research team

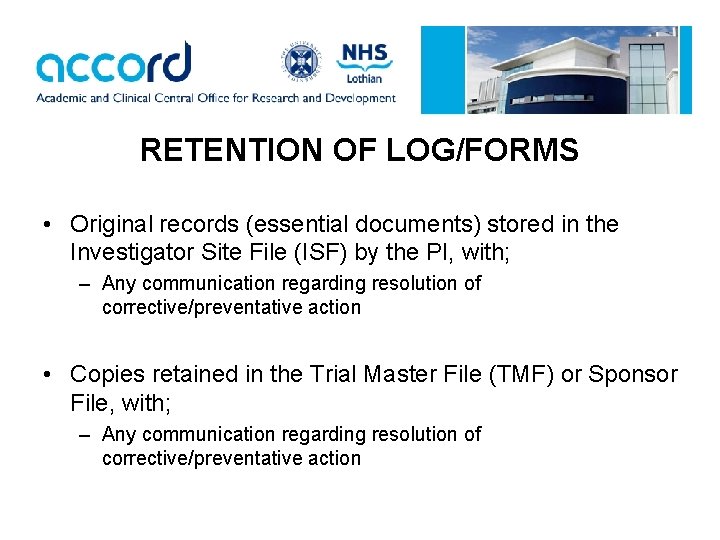
RETENTION OF LOG/FORMS • Original records (essential documents) stored in the Investigator Site File (ISF) by the PI, with; – Any communication regarding resolution of corrective/preventative action • Copies retained in the Trial Master File (TMF) or Sponsor File, with; – Any communication regarding resolution of corrective/preventative action

CONTACTS & REFERENCES • Please address any questions to; – QA@accord. scot – Lorn. Mackenzie@nhslothian. scot. nhs. uk (QA Manager) – Gavin. Robertson@nhslothian. scot. nhs. uk (QA Coordinator) • SOP CR 010 and examples of completed Deviation Logs/Violations Forms are available on the ACCORD website – www. accord. scot