Management of Coronary Artery Disease Saravanan Kuppuswamy MD














































- Slides: 99

Management of Coronary Artery Disease: Saravanan Kuppuswamy MD Division of Cardiology Department of Internal Medicine University of Missouri Hospital


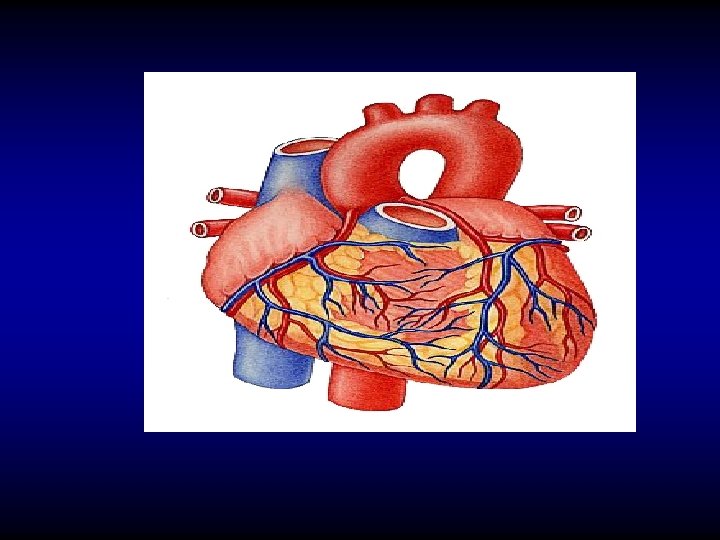
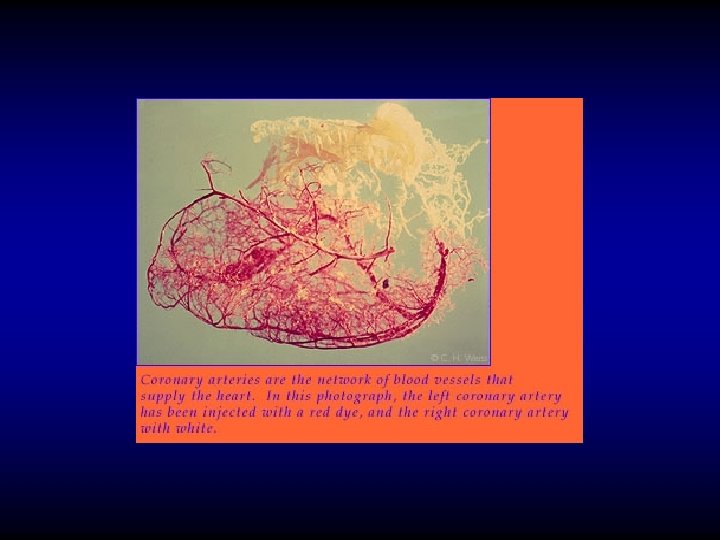
Coronary blood supply

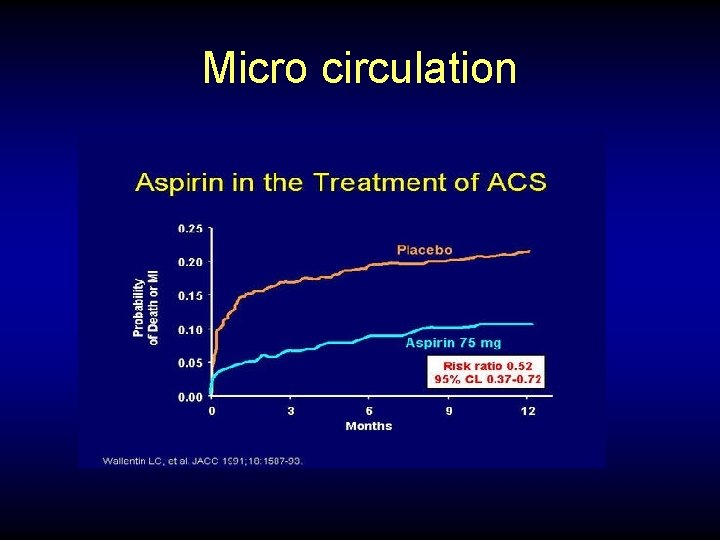
Micro circulation




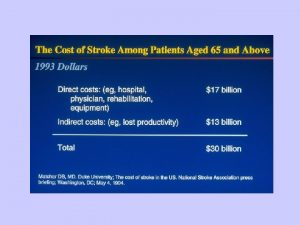
Temporal Trends in CAD • CHD is the leading cause of death in adults in US (1/3 of all deaths in subjects over age 35) • Mortality rates for cardiovascular death has fallen in most developed countries 24 -28% since 1975 • Estimated that 45 percent of mortality reduction in CHD is due to improvement of medical therapy and 55% is due to risk factor modification

Etiology • Congenital • Acquired – Infection – Inflammation – Neoplastic

Management of CAD • Acute • Chronic

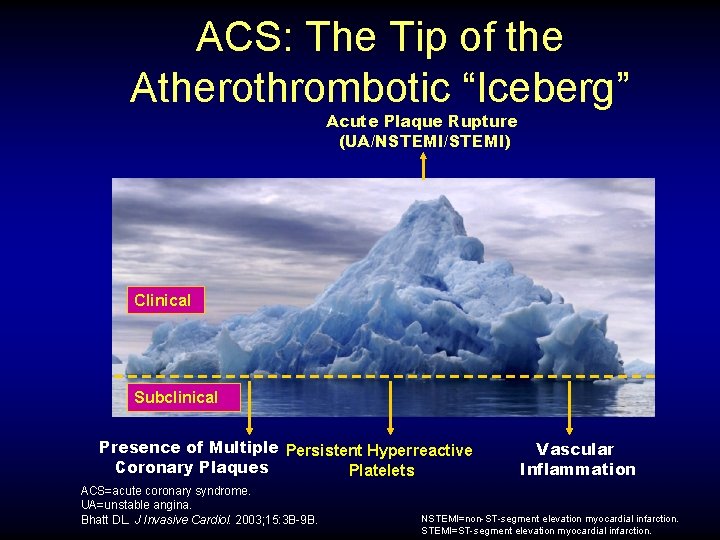
ACS: The Tip of the Atherothrombotic “Iceberg” Acute Plaque Rupture (UA/NSTEMI/STEMI) Clinical Subclinical Presence of Multiple Persistent Hyperreactive Coronary Plaques Platelets ACS=acute coronary syndrome. UA=unstable angina. Bhatt DL. J Invasive Cardiol. 2003; 15: 3 B-9 B. Vascular Inflammation NSTEMI=non-ST-segment elevation myocardial infarction. STEMI=ST-segment elevation myocardial infarction.

Chronic stable angina • Levine’s sign • Exercise capacity may vary • Relieved by nitrates

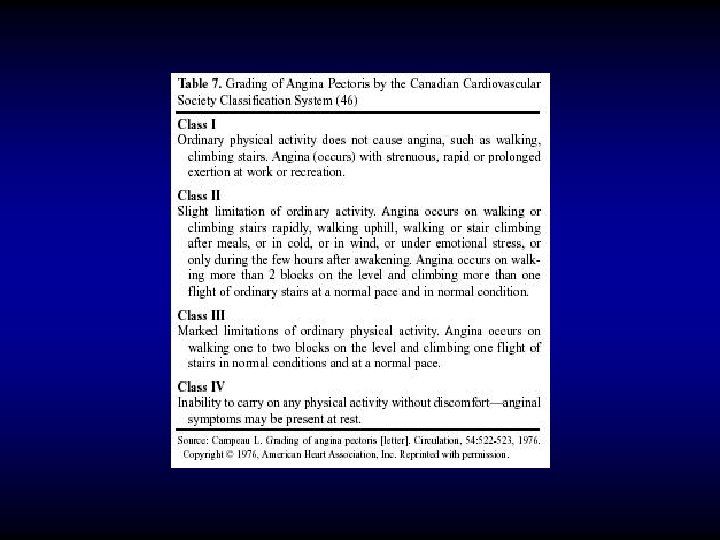
CCS classification


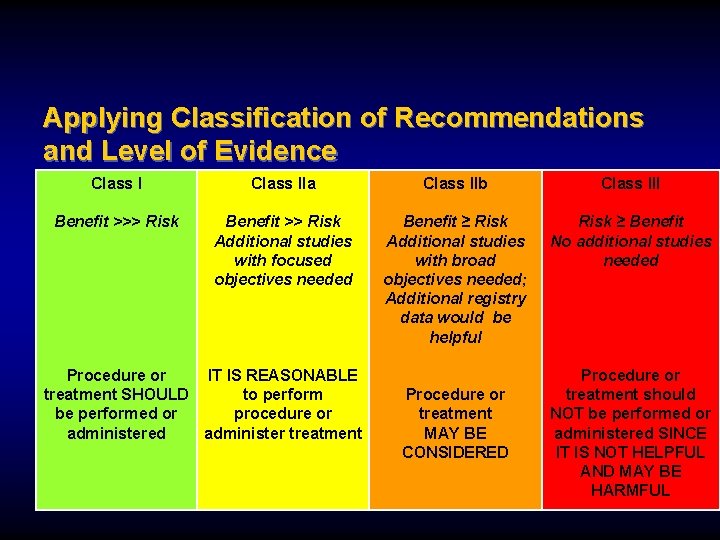
Applying Classification of Recommendations and Level of Evidence Class IIa Class IIb Class III Benefit >>> Risk Benefit >> Risk Additional studies with focused objectives needed Benefit ≥ Risk Additional studies with broad objectives needed; Additional registry data would be helpful Risk ≥ Benefit No additional studies needed Procedure or IT IS REASONABLE treatment SHOULD to perform be performed or procedure or administered administer treatment Procedure or treatment MAY BE CONSIDERED Procedure or treatment should NOT be performed or administered SINCE IT IS NOT HELPFUL AND MAY BE HARMFUL

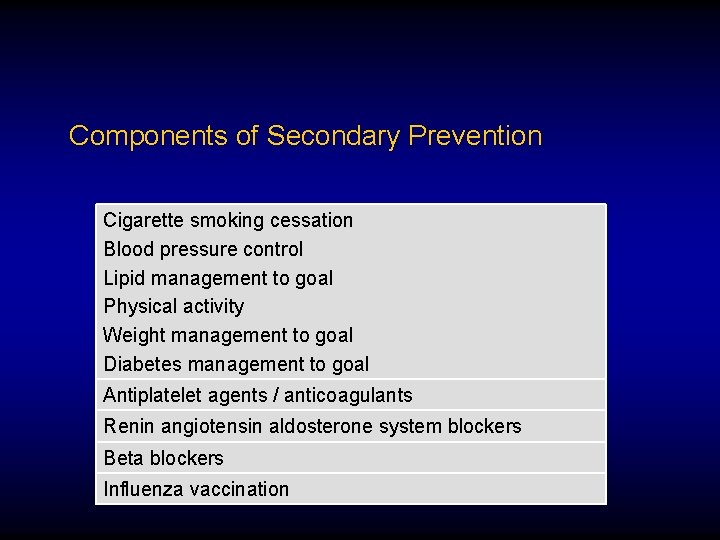
Components of Secondary Prevention Cigarette smoking cessation Blood pressure control Lipid management to goal Physical activity Weight management to goal Diabetes management to goal Antiplatelet agents / anticoagulants Renin angiotensin aldosterone system blockers Beta blockers Influenza vaccination

ABCDE Of Management

A

Antiplatelet Agents / Anticoagulation Recommendations

Aspirin Recommendations Start and continue indefinitely aspirin 75 to 162 mg/d in all patients unless contraindicated For patients undergoing CABG, aspirin (100 to 325 mg/d) should be started within 48 hours after surgery to reduce saphenous vein graft closure Post-PCI-stented patients should receive 325 mg per day of aspirin for 1 month for bare metal stent, 3 months for sirolimus-eluting stent and 6 months for paclitaxel-eluting stent

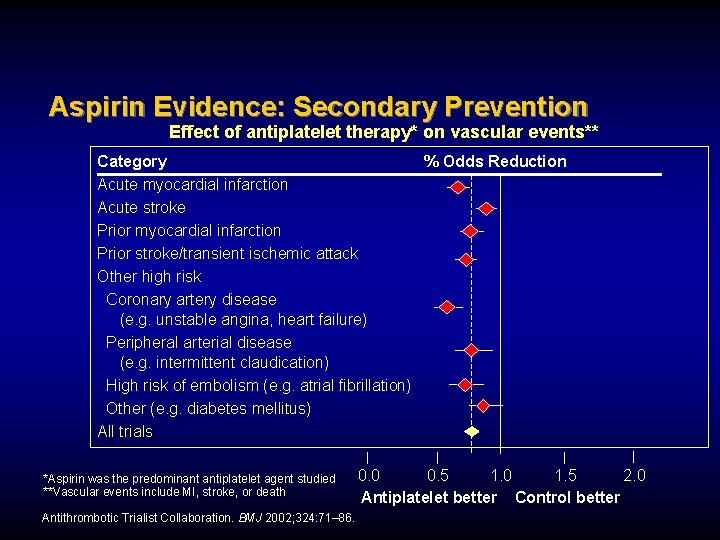
Aspirin Evidence: Secondary Prevention Effect of antiplatelet therapy* on vascular events** Category % Odds Reduction Acute myocardial infarction Acute stroke Prior myocardial infarction Prior stroke/transient ischemic attack Other high risk Coronary artery disease (e. g. unstable angina, heart failure) Peripheral arterial disease (e. g. intermittent claudication) High risk of embolism (e. g. atrial fibrillation) Other (e. g. diabetes mellitus) All trials *Aspirin was the predominant antiplatelet agent studied **Vascular events include MI, stroke, or death Antithrombotic Trialist Collaboration. BMJ 2002; 324: 71– 86. 0. 0 0. 5 1. 0 1. 5 2. 0 Antiplatelet better Control better

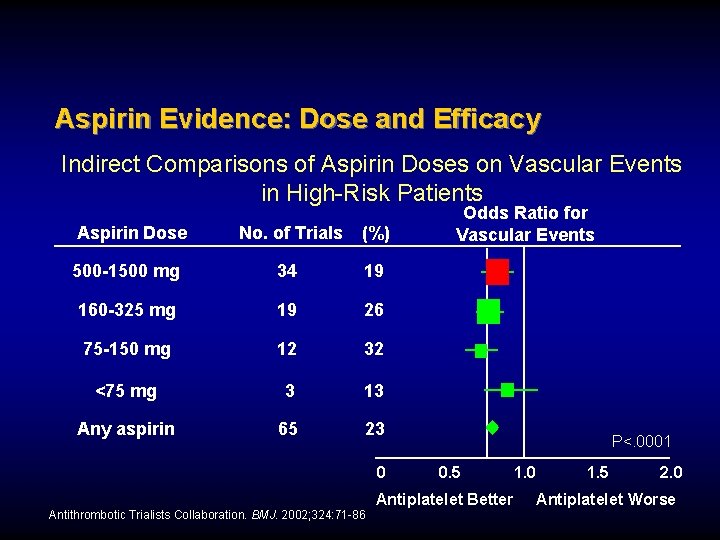
Aspirin Evidence: Dose and Efficacy Indirect Comparisons of Aspirin Doses on Vascular Events in High-Risk Patients Aspirin Dose No. of Trials (%) 500 -1500 mg 34 19 160 -325 mg 19 26 75 -150 mg 12 32 <75 mg 3 13 Any aspirin 65 23 0 Odds Ratio for Vascular Events P<. 0001 0. 5 Antiplatelet Better Antithrombotic Trialists Collaboration. BMJ. 2002; 324: 71 -86 1. 0 1. 5 2. 0 Antiplatelet Worse

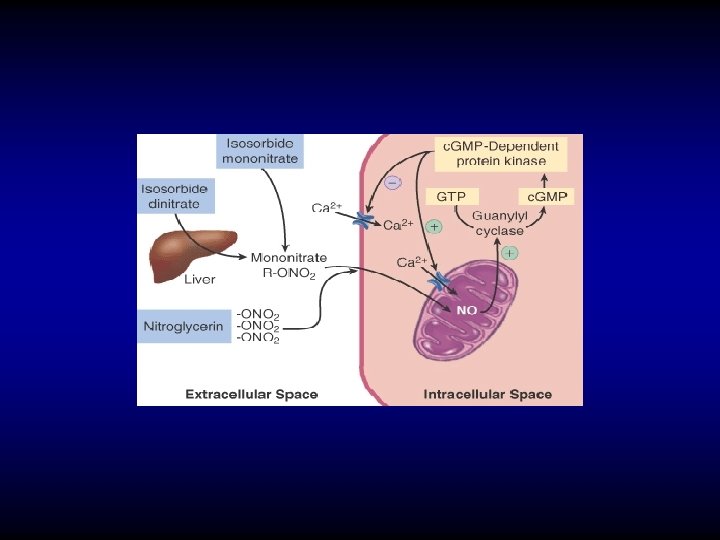
Mechanism of action of nitrates

B

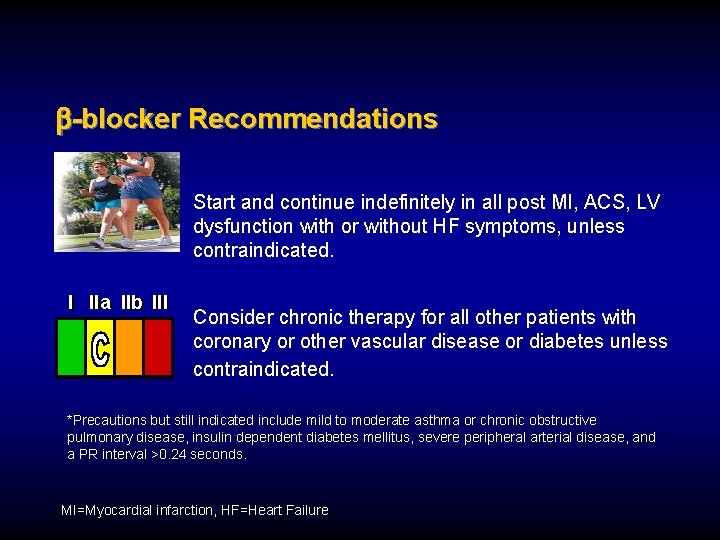
b-blocker Recommendations



-blocker Recommendations Start and continue indefinitely in all post MI, ACS, LV dysfunction with or without HF symptoms, unless contraindicated. I IIa IIb III Consider chronic therapy for all other patients with coronary or other vascular disease or diabetes unless contraindicated. *Precautions but still indicated include mild to moderate asthma or chronic obstructive pulmonary disease, insulin dependent diabetes mellitus, severe peripheral arterial disease, and a PR interval >0. 24 seconds. MI=Myocardial infarction, HF=Heart Failure

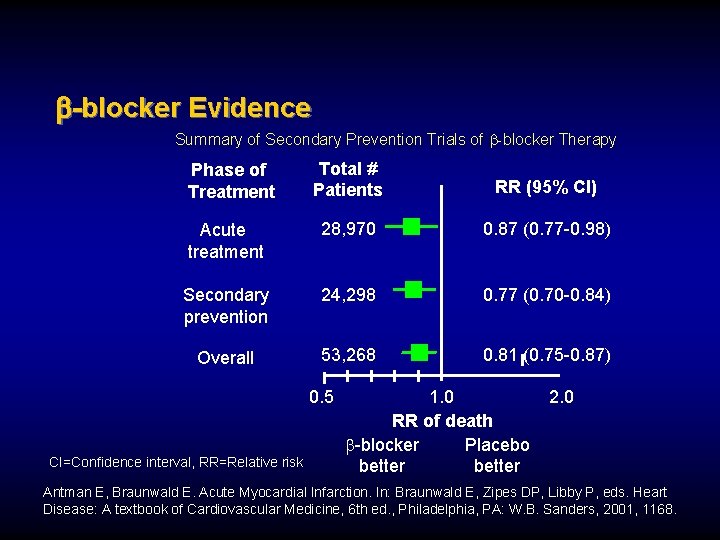
-blocker Evidence Summary of Secondary Prevention Trials of b-blocker Therapy Total # Patients RR (95% CI) Acute treatment 28, 970 0. 87 (0. 77 -0. 98) Secondary prevention 24, 298 0. 77 (0. 70 -0. 84) Overall 53, 268 0. 81 (0. 75 -0. 87) Phase of Treatment 0. 5 CI=Confidence interval, RR=Relative risk 1. 0 RR of death b-blocker Placebo better 2. 0 Antman E, Braunwald E. Acute Myocardial Infarction. In: Braunwald E, Zipes DP, Libby P, eds. Heart Disease: A textbook of Cardiovascular Medicine, 6 th ed. , Philadelphia, PA: W. B. Sanders, 2001, 1168.

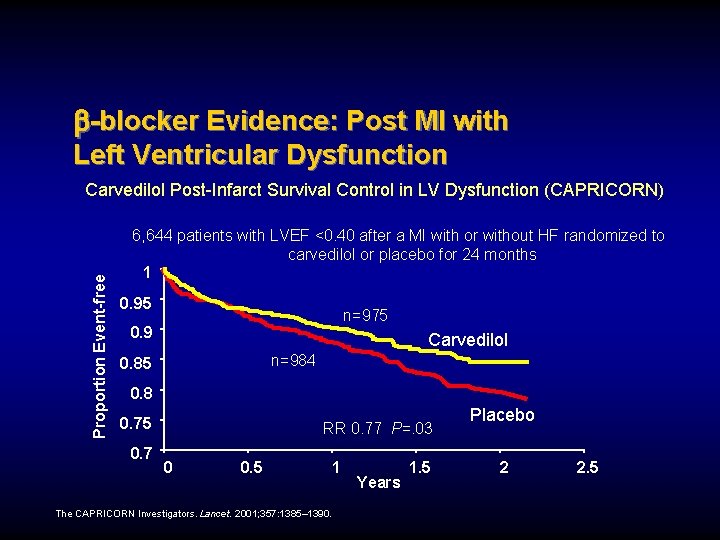
-blocker Evidence: Post MI with Left Ventricular Dysfunction Proportion Event-free Carvedilol Post-Infarct Survival Control in LV Dysfunction (CAPRICORN) 6, 644 patients with LVEF <0. 40 after a MI with or without HF randomized to carvedilol or placebo for 24 months 1 0. 95 n=975 0. 9 Carvedilol n=984 0. 85 0. 8 0. 75 0. 7 RR 0. 77 P=. 03 0 0. 5 1 The CAPRICORN Investigators. Lancet. 2001; 357: 1385– 1390. Years 1. 5 Placebo 2 2. 5

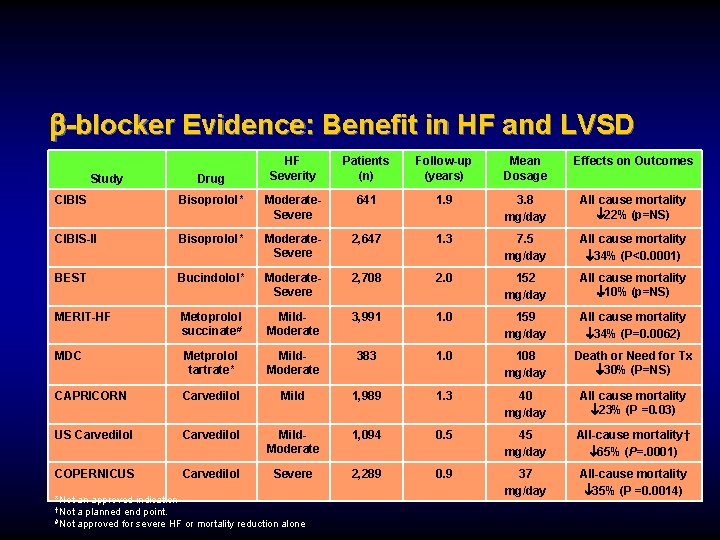
-blocker Evidence: Benefit in HF and LVSD Study Drug HF Severity Patients (n) Follow-up (years) Mean Dosage Effects on Outcomes CIBIS Bisoprolol* Moderate. Severe 641 1. 9 3. 8 mg/day All cause mortality 22% (p=NS) CIBIS-II Bisoprolol* Moderate. Severe 2, 647 1. 3 7. 5 mg/day All cause mortality 34% (P<0. 0001) BEST Bucindolol* Moderate. Severe 2, 708 2. 0 152 mg/day All cause mortality 10% (p=NS) MERIT-HF Metoprolol succinate# Mild. Moderate 3, 991 1. 0 159 mg/day All cause mortality 34% (P=0. 0062) MDC Metprolol tartrate* Mild. Moderate 383 1. 0 108 mg/day Death or Need for Tx 30% (P=NS) CAPRICORN Carvedilol Mild 1, 989 1. 3 40 mg/day All cause mortality 23% (P =0. 03) US Carvedilol Mild. Moderate 1, 094 0. 5 45 mg/day All-cause mortality† 65% (P=. 0001) COPERNICUS Carvedilol Severe 2, 289 0. 9 37 mg/day All-cause mortality 35% (P =0. 0014) *Not an approved indication †Not a planned end point. #Not approved for severe HF or mortality reduction alone

Blood Pressure Control Recommendations Goal: <140/90 mm Hg or <130/80 if diabetes or chronic kidney disease Blood pressure 120/80 mm Hg or greater: · Initiate or maintain lifestyle modification: weight control, increased physical activity, alcohol moderation, sodium reduction, and increased consumption of fresh fruits vegetables and low fat dairy products Blood pressure 140/90 mm Hg or greater (or 130/80 or greater for chronic kidney disease or diabetes) · As tolerated, add blood pressure medication, treating initially with beta blockers and/or ACE inhibitors with addition of other drugs such as thiazides as needed to achieve goal blood pressure

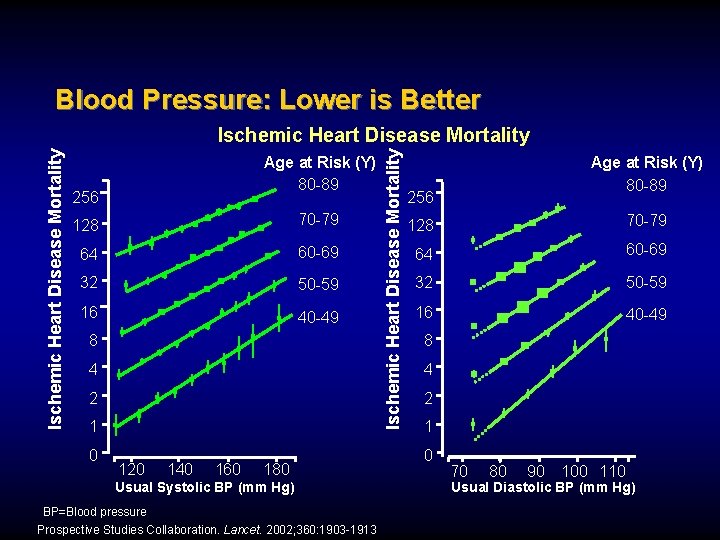
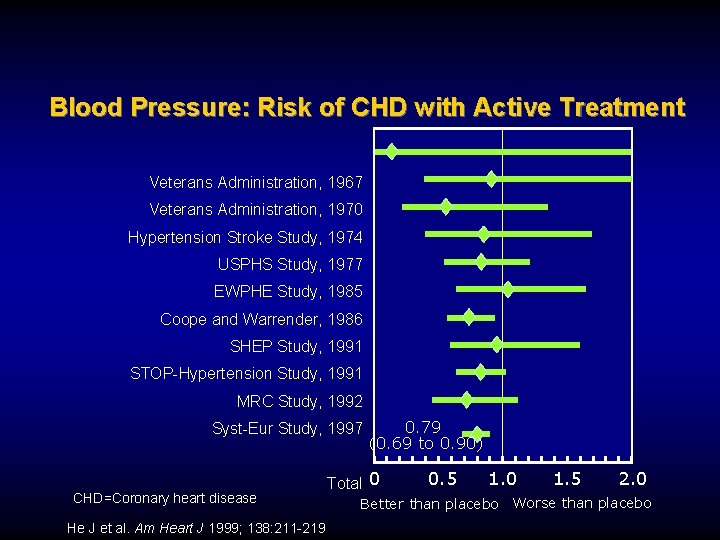
Blood Pressure: Lower is Better Age at Risk (Y) 80 -89 256 128 70 -79 64 60 -69 32 50 -59 16 40 -49 8 4 2 1 0 120 140 160 180 Usual Systolic BP (mm Hg) BP=Blood pressure Prospective Studies Collaboration. Lancet. 2002; 360: 1903 -1913 Ischemic Heart Disease Mortality Age at Risk (Y) 80 -89 256 128 70 -79 64 60 -69 32 50 -59 16 40 -49 8 4 2 1 0 70 80 90 100 110 Usual Diastolic BP (mm Hg)

Blood Pressure: Risk of CHD with Active Treatment Veterans Administration, 1967 Veterans Administration, 1970 Hypertension Stroke Study, 1974 USPHS Study, 1977 EWPHE Study, 1985 Coope and Warrender, 1986 SHEP Study, 1991 STOP-Hypertension Study, 1991 MRC Study, 1992 Syst-Eur Study, 1997 CHD=Coronary heart disease He J et al. Am Heart J 1999; 138: 211 -219 0. 79 (0. 69 to 0. 90) Total 0 0. 5 1. 0 1. 5 2. 0 Better than placebo Worse than placebo

JNC VII Guidelines for Management and Treatment SBP* BP classification mm. Hg Normal DBP* Lifestyle mm. Hg modification <120 <80 Encourage Prehypertension 120– 139 80– 89 Yes Stage 1 Hypertension 140– 159 90– 99 Yes Stage 2 Hypertension >160 >100 Yes Initial drug therapy With compelling indications Drug(s) for compelling indications. ‡ Drug(s) for the compelling indications. ‡ Other antihypertensive drugs (diuretics, ACEI, ARB, BB, CCB) as needed. ACEI=Angiotensin converting enzyme inhibitor, ARB=Angiotensin receptor blocker, BB=b-blocker, BP=Blood pressure, CCB=Calcium channel blocker, DBP=Diastolic blood pressure, SBP=Systolic blood pressure *Treatment determined by highest blood pressure category. †Initial combined therapy should be used cautiously in those at risk for orthostatic hypotension. ‡Treat patients with chronic kidney disease or diabetes mellitus to blood pressure goal of <130/80 mm. Hg. Chobanian AV et al. JAMA. 2003; 289: 2560 -2572

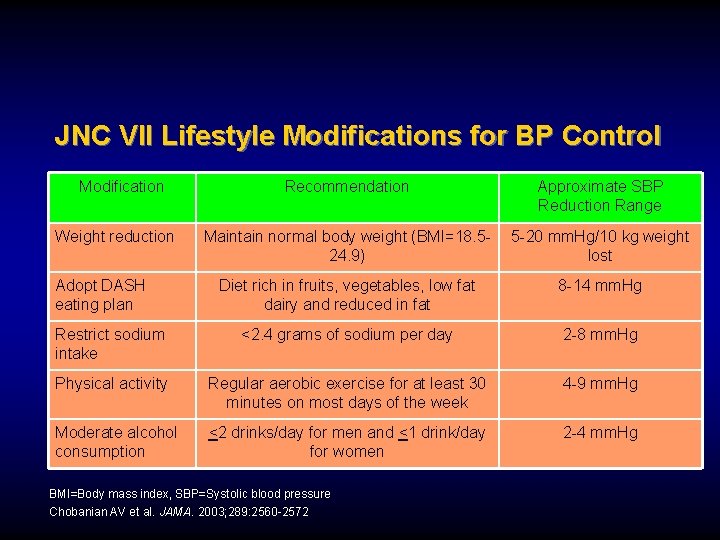
JNC VII Lifestyle Modifications for BP Control Modification Recommendation Approximate SBP Reduction Range Maintain normal body weight (BMI=18. 524. 9) 5 -20 mm. Hg/10 kg weight lost Diet rich in fruits, vegetables, low fat dairy and reduced in fat 8 -14 mm. Hg Restrict sodium intake <2. 4 grams of sodium per day 2 -8 mm. Hg Physical activity Regular aerobic exercise for at least 30 minutes on most days of the week 4 -9 mm. Hg Moderate alcohol consumption <2 drinks/day for men and <1 drink/day for women 2 -4 mm. Hg Weight reduction Adopt DASH eating plan BMI=Body mass index, SBP=Systolic blood pressure Chobanian AV et al. JAMA. 2003; 289: 2560 -2572

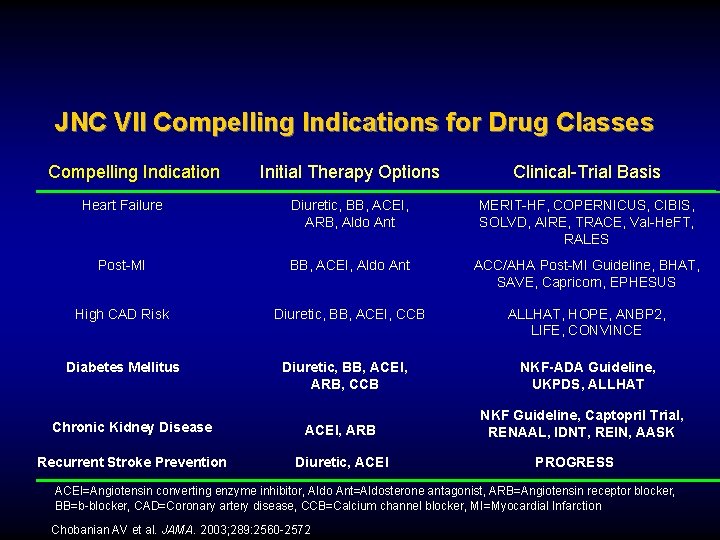
JNC VII Compelling Indications for Drug Classes Compelling Indication Initial Therapy Options Clinical-Trial Basis Heart Failure Diuretic, BB, ACEI, ARB, Aldo Ant MERIT-HF, COPERNICUS, CIBIS, SOLVD, AIRE, TRACE, Val-He. FT, RALES Post-MI BB, ACEI, Aldo Ant ACC/AHA Post-MI Guideline, BHAT, SAVE, Capricorn, EPHESUS High CAD Risk Diuretic, BB, ACEI, CCB ALLHAT, HOPE, ANBP 2, LIFE, CONVINCE Diabetes Mellitus Diuretic, BB, ACEI, ARB, CCB Chronic Kidney Disease ACEI, ARB Recurrent Stroke Prevention Diuretic, ACEI NKF-ADA Guideline, UKPDS, ALLHAT NKF Guideline, Captopril Trial, RENAAL, IDNT, REIN, AASK PROGRESS ACEI=Angiotensin converting enzyme inhibitor, Aldo Ant=Aldosterone antagonist, ARB=Angiotensin receptor blocker, BB=b-blocker, CAD=Coronary artery disease, CCB=Calcium channel blocker, MI=Myocardial Infarction Chobanian AV et al. JAMA. 2003; 289: 2560 -2572

C

Cigarette Smoking Recommendations Goal: Complete Cessation and No Exposure to Environmental Tobacco Smoke • Ask about tobacco use status at every visit. • Advise every tobacco user to quit. • Assess the tobacco user’s willingness to quit. • Assist by counseling and developing a plan for quitting. • Arrange follow-up, referral to special programs, or pharmacotherapy (including nicotine replacement and bupropion. • Urge avoidance of exposure to environmental tobacco smoke at work and home.

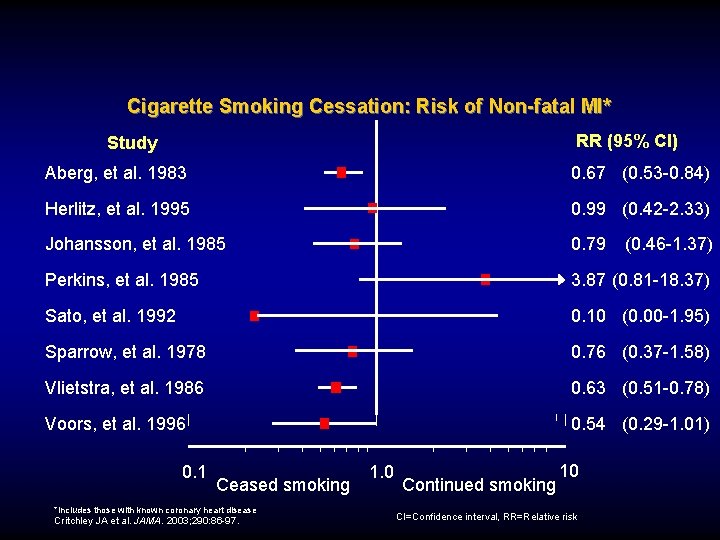
Cigarette Smoking Cessation: Risk of Non-fatal MI* RR (95% Cl) Study Aberg, et al. 1983 0. 67 (0. 53 -0. 84) Herlitz, et al. 1995 0. 99 (0. 42 -2. 33) Johansson, et al. 1985 0. 79 (0. 46 -1. 37) Perkins, et al. 1985 3. 87 (0. 81 -18. 37) Sato, et al. 1992 0. 10 (0. 00 -1. 95) Sparrow, et al. 1978 0. 76 (0. 37 -1. 58) Vlietstra, et al. 1986 0. 63 (0. 51 -0. 78) Voors, et al. 1996 0. 54 (0. 29 -1. 01) 0. 1 Ceased smoking *Includes those with known coronary heart disease Critchley JA et al. JAMA. 2003; 290: 86 -97. 1. 0 Continued smoking 10 CI=Confidence interval, RR=Relative risk

Lipid Management Goals: NCEP Risk Category High risk: CHD or CHD risk equivalents (10 -year risk >20%) and Very high risk: ACS or established CHD plus: multiple major risk factors (especially diabetes) or severe and poorly controlled risk factors Consider Drug Therapy LDL-C and non-HDLC Goal Initiate TLC <100 mg/d. L if TG > 200 mg/d. L, non-HDL-C should be < 130 mg/d. L 100 mg/d. L >100 mg/d. L (<100 mg/d. L: consider drug options) <70 mg/d. L, non-HDL-C < 100 mg/d. L All patients >100 mg/d. L (<100 mg/d. L: consider drug options) ATP=Adult Treatment Panel, CHD=Coronary heart disease, LDL-C=Low-density lipoprotein cholesterol, TLC=Therapeutic lifestyle changes Grundy, S. et al. Circulation 2004; 110: 227 -39.

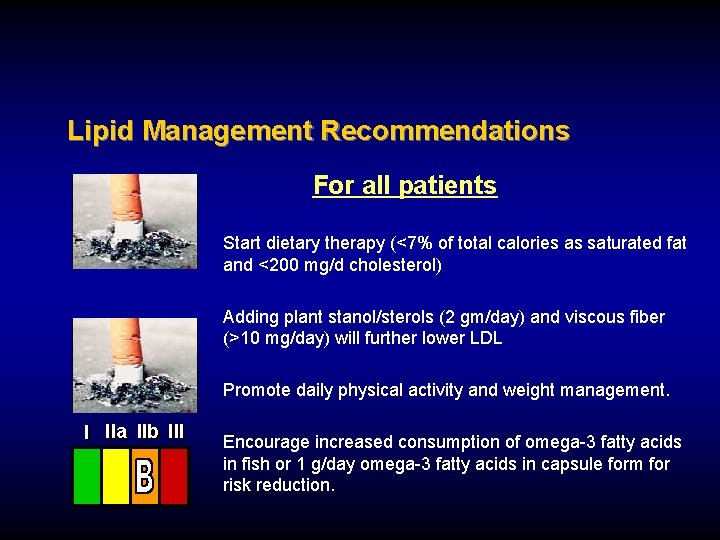
Lipid Management Recommendations For all patients Start dietary therapy (<7% of total calories as saturated fat and <200 mg/d cholesterol) Adding plant stanol/sterols (2 gm/day) and viscous fiber (>10 mg/day) will further lower LDL Promote daily physical activity and weight management. I IIa IIb III Encourage increased consumption of omega-3 fatty acids in fish or 1 g/day omega-3 fatty acids in capsule form for risk reduction.

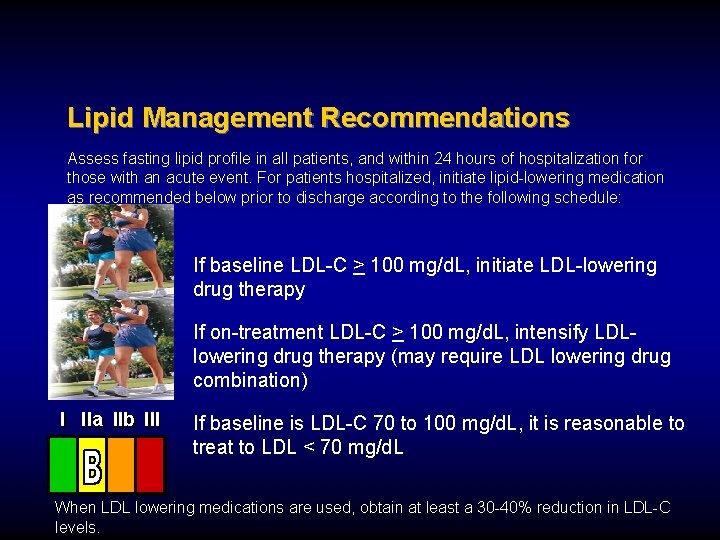
Lipid Management Recommendations Assess fasting lipid profile in all patients, and within 24 hours of hospitalization for those with an acute event. For patients hospitalized, initiate lipid-lowering medication as recommended below prior to discharge according to the following schedule: If baseline LDL-C > 100 mg/d. L, initiate LDL-lowering drug therapy If on-treatment LDL-C > 100 mg/d. L, intensify LDLlowering drug therapy (may require LDL lowering drug combination) I IIa IIb III If baseline is LDL-C 70 to 100 mg/d. L, it is reasonable to treat to LDL < 70 mg/d. L When LDL lowering medications are used, obtain at least a 30 -40% reduction in LDL-C levels.

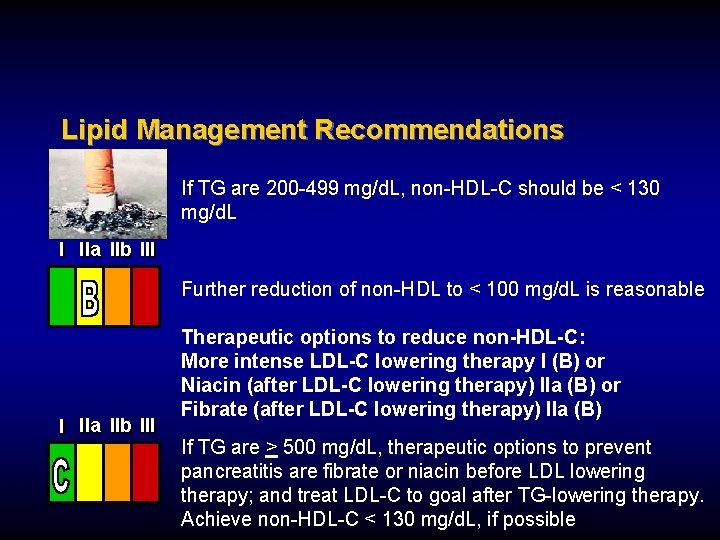
Lipid Management Recommendations If TG are 200 -499 mg/d. L, non-HDL-C should be < 130 mg/d. L I IIa IIb III Further reduction of non-HDL to < 100 mg/d. L is reasonable I IIa IIb III Therapeutic options to reduce non-HDL-C: More intense LDL-C lowering therapy I (B) or Niacin (after LDL-C lowering therapy) IIa (B) or Fibrate (after LDL-C lowering therapy) IIa (B) If TG are > 500 mg/d. L, therapeutic options to prevent pancreatitis are fibrate or niacin before LDL lowering therapy; and treat LDL-C to goal after TG-lowering therapy. Achieve non-HDL-C < 130 mg/d. L, if possible

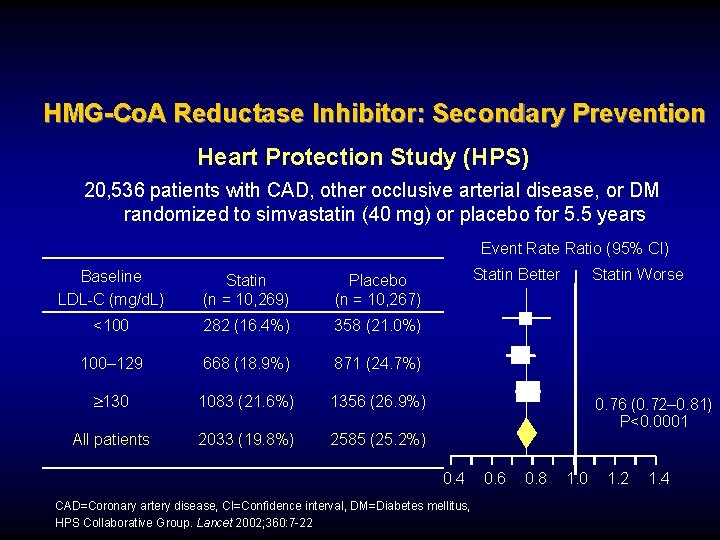
HMG-Co. A Reductase Inhibitor: Secondary Prevention Heart Protection Study (HPS) 20, 536 patients with CAD, other occlusive arterial disease, or DM randomized to simvastatin (40 mg) or placebo for 5. 5 years Event Rate Ratio (95% CI) Baseline LDL-C (mg/d. L) Statin (n = 10, 269) Placebo (n = 10, 267) <100 282 (16. 4%) 358 (21. 0%) 100– 129 668 (18. 9%) 871 (24. 7%) 130 1083 (21. 6%) 1356 (26. 9%) All patients 2033 (19. 8%) 2585 (25. 2%) Statin Better Statin Worse 0. 76 (0. 72– 0. 81) P<0. 0001 0. 4 CAD=Coronary artery disease, CI=Confidence interval, DM=Diabetes mellitus, HPS Collaborative Group. Lancet 2002; 360: 7 -22 0. 6 0. 8 1. 0 1. 2 1. 4

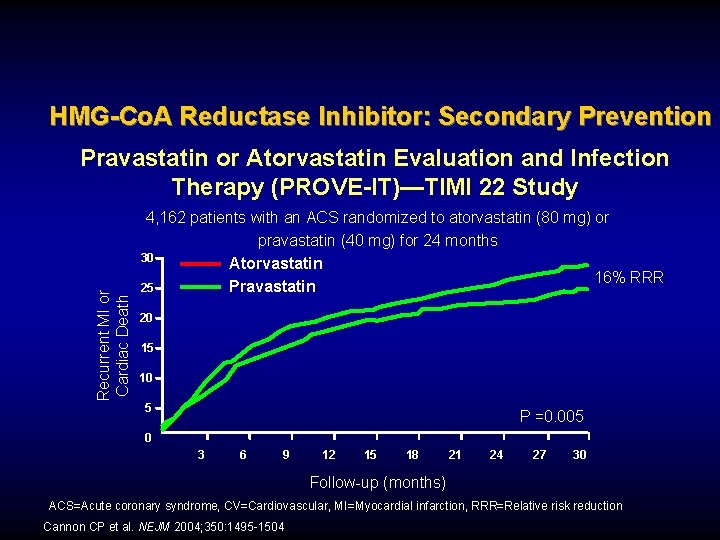
HMG-Co. A Reductase Inhibitor: Secondary Prevention Recurrent MI or Cardiac Death Pravastatin or Atorvastatin Evaluation and Infection Therapy (PROVE-IT)—TIMI 22 Study 4, 162 patients with an ACS randomized to atorvastatin (80 mg) or pravastatin (40 mg) for 24 months 30 Atorvastatin 16% RRR 25 Pravastatin 20 15 10 5 P =0. 005 0 3 6 9 12 15 18 21 24 27 30 Follow-up (months) ACS=Acute coronary syndrome, CV=Cardiovascular, MI=Myocardial infarction, RRR=Relative risk reduction Cannon CP et al. NEJM 2004; 350: 1495 -1504

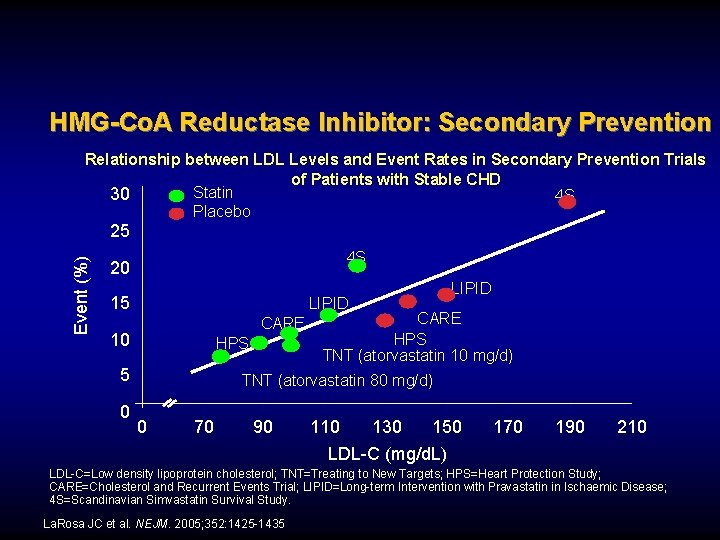
HMG-Co. A Reductase Inhibitor: Secondary Prevention Relationship between LDL Levels and Event Rates in Secondary Prevention Trials of Patients with Stable CHD Statin 30 4 S Placebo Event (%) 25 4 S 20 15 LIPID CARE 10 HPS 5 0 LIPID CARE HPS TNT (atorvastatin 10 mg/d) TNT (atorvastatin 80 mg/d) 0 70 90 110 130 150 LDL-C (mg/d. L) 170 190 210 LDL-C=Low density lipoprotein cholesterol; TNT=Treating to New Targets; HPS=Heart Protection Study; CARE=Cholesterol and Recurrent Events Trial; LIPID=Long-term Intervention with Pravastatin in Ischaemic Disease; 4 S=Scandinavian Simvastatin Survival Study. La. Rosa JC et al. NEJM. 2005; 352: 1425 -1435

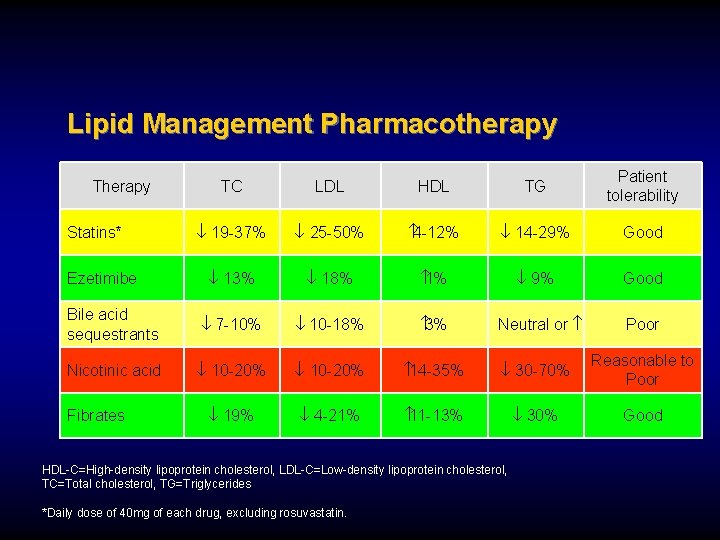
Lipid Management Pharmacotherapy TC LDL HDL TG Patient tolerability ¯ 19 -37% ¯ 25 -50% 4 -12% ¯ 14 -29% Good ¯ 13% ¯ 18% 1% ¯ 9% Good Bile acid sequestrants ¯ 7 -10% ¯ 10 -18% 3% Nicotinic acid ¯ 10 -20% 14 -35% ¯ 30 -70% Reasonable to Poor ¯ 19% ¯ 4 -21% 11 -13% ¯ 30% Good Therapy Statins* Ezetimibe Fibrates Neutral or HDL-C=High-density lipoprotein cholesterol, LDL-C=Low-density lipoprotein cholesterol, TC=Total cholesterol, TG=Triglycerides *Daily dose of 40 mg of each drug, excluding rosuvastatin. Poor

Lipid Management Goal LDL-C should be less than 100 mg/d. L I IIa IIb III Further reduction to LDL-C to < 70 mg/d. L is reasonable If TG >200 mg/d. L, non-HDL-C should be < 130 mg/d. L* *Non-HDL-C = total cholesterol minus HDL-C

D

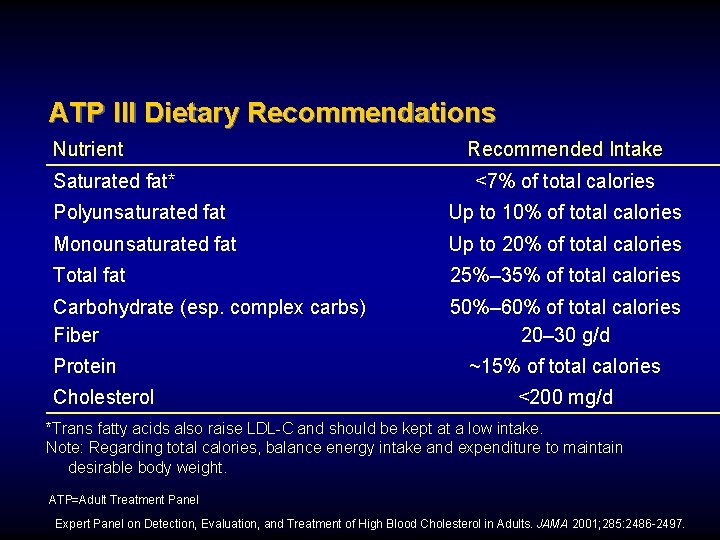
ATP III Dietary Recommendations Nutrient Saturated fat* Recommended Intake <7% of total calories Polyunsaturated fat Up to 10% of total calories Monounsaturated fat Up to 20% of total calories Total fat 25%– 35% of total calories Carbohydrate (esp. complex carbs) Fiber 50%– 60% of total calories 20– 30 g/d Protein Cholesterol ~15% of total calories <200 mg/d *Trans fatty acids also raise LDL-C and should be kept at a low intake. Note: Regarding total calories, balance energy intake and expenditure to maintain desirable body weight. ATP=Adult Treatment Panel Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. JAMA 2001; 285: 2486 -2497.

Weight Management Recommendations Goal: BMI 18. 5 to 24. 9 kg/m 2 Waist Circumference: Men: < 40 inches Women: < 35 inches Assess BMI and/or waist circumference on each visit and consistently encourage weight maintenance/ reduction through an appropriate balance of physical activity, caloric intake, and formal behavioral programs when indicated. If waist circumference (measured at the iliac crest) >35 inches in women and >40 inches in men initiate lifestyle changes and consider treatment strategies for metabolic syndrome as indicated. The initial goal of weight loss therapy should be to reduce body weight by approximately 10 percent from baseline. With success, further weight loss can be attempted if indicated. *BMI is calculated as the weight in kilograms divided by the body surface area in meters 2. Overweight state is defined by BMI=25 -30 kg/m 2. Obesity is defined by a BMI >30 kg/m 2.

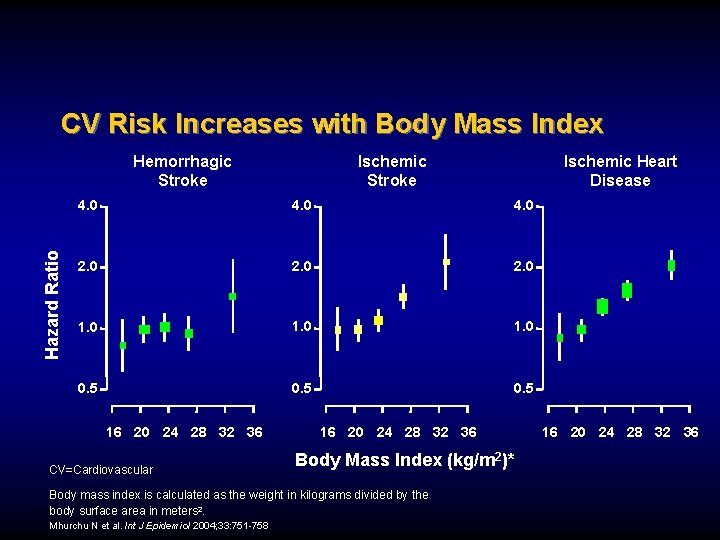
CV Risk Increases with Body Mass Index Hazard Ratio Hemorrhagic Stroke Ischemic Heart Disease 4. 0 2. 0 1. 0 0. 5 16 20 24 28 32 36 CV=Cardiovascular 16 20 24 28 32 36 Body Mass Index (kg/m 2)* Body mass index is calculated as the weight in kilograms divided by the body surface area in meters 2. Mhurchu N et al. Int J Epidemiol 2004; 33: 751 -758 16 20 24 28 32 36

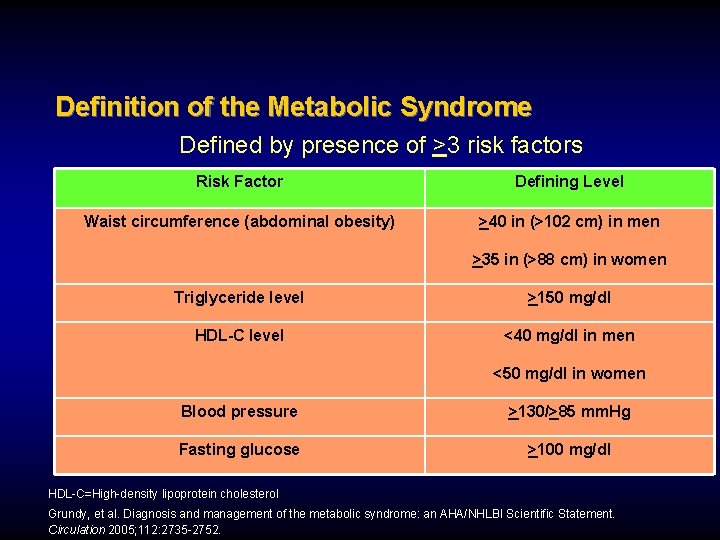
Definition of the Metabolic Syndrome Defined by presence of >3 risk factors Risk Factor Defining Level Waist circumference (abdominal obesity) >40 in (>102 cm) in men >35 in (>88 cm) in women Triglyceride level >150 mg/dl HDL-C level <40 mg/dl in men <50 mg/dl in women Blood pressure >130/>85 mm. Hg Fasting glucose >100 mg/dl HDL-C=High-density lipoprotein cholesterol Grundy, et al. Diagnosis and management of the metabolic syndrome: an AHA/NHLBI Scientific Statement. Circulation 2005; 112: 2735 -2752.

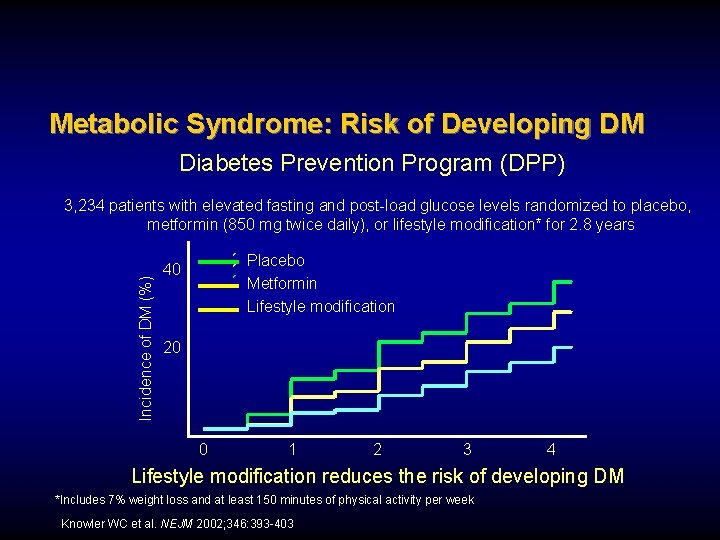
Metabolic Syndrome: Risk of Developing DM Diabetes Prevention Program (DPP) Incidence of DM (%) 3, 234 patients with elevated fasting and post-load glucose levels randomized to placebo, metformin (850 mg twice daily), or lifestyle modification* for 2. 8 years Placebo Metformin Lifestyle modification 40 20 0 1 2 3 4 Lifestyle modification reduces the risk of developing DM *Includes 7% weight loss and at least 150 minutes of physical activity per week Knowler WC et al. NEJM 2002; 346: 393 -403

Diabetes Mellitus Recommendations Goal: Hb A 1 c < 7% Lifestyle and pharmacotherapy to achieve near normal Hb. A 1 C (<7%). I IIa IIb III Vigorous modification of other risk factors (e. g. , physical activity, weight management, blood pressure control, and cholesterol management as recommended). Coordinate diabetic care with patient’s primary care physician or endocrinologist. ) Hb. A 1 c = Glycosylated hemoglobin

E

Physical Activity Recommendations Goal: 30 minutes 7 days/week, minimum 5 days/week Assess risk with a physical activity history and/or an exercise test, to guide prescription Encourage 30 to 60 minutes of moderate intensity aerobic activity such as brisk walking, on most, preferably all, days of the week, supplemented by an increase in daily lifestyle activities Advise medically supervised programs for high-risk patients (e. g. recent acute coronary syndrome or revascularization,

Exercise Evidence: Mortality Risk Observational study of self-reported physical activity in 772 men with established coronary heart disease Light or moderate exercise is associated with lower risk Wannamethee SG et al. Circulation 2000; 102: 1358 -1363


Renin-Angiotensin-Aldosterone System Blockers Recommendations

ACE Inhibitor Recommendations Use in all patients with LVEF < 40%, and those with diabetes or chronic kidney disease indefinitely, unless contraindicated Consider for all other patients I IIa IIb III Among lower risk patients with normal LVEF where cardiovascular risk factors are well controlled and where revascularization has been performed, their use may be considered optional ACE=Angiotensin converting enzyme, LVEF= left ventricular ejection fraction




STRIVE TM

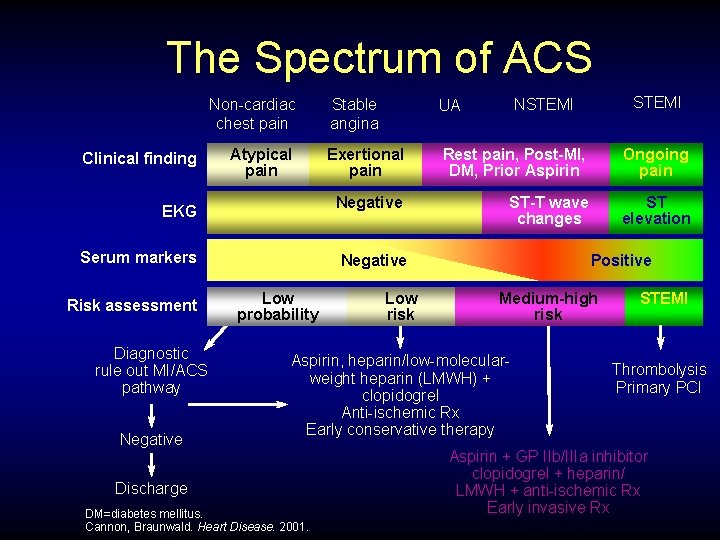
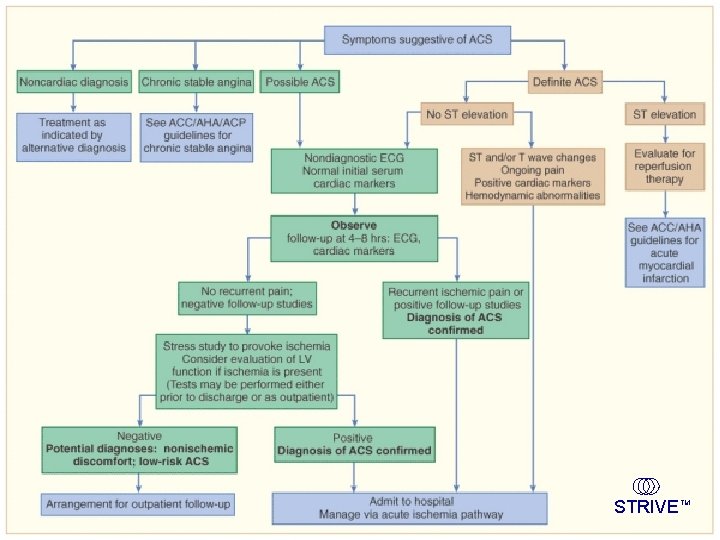
The Spectrum of ACS Non-cardiac chest pain Clinical finding Atypical pain EKG Serum markers Risk assessment Diagnostic rule out MI/ACS pathway Negative UA NSTEMI Exertional pain Rest pain, Post-MI, DM, Prior Aspirin Ongoing pain Negative ST-T wave changes ST elevation Negative Low probability Low risk Positive Medium-high risk Aspirin, heparin/low-molecularweight heparin (LMWH) + clopidogrel Anti-ischemic Rx Early conservative therapy Discharge DM=diabetes mellitus. Cannon, Braunwald. Heart Disease. 2001. STEMI Stable angina STEMI Thrombolysis Primary PCI Aspirin + GP IIb/IIIa inhibitor clopidogrel + heparin/ LMWH + anti-ischemic Rx Early invasive Rx

Acute Coronary Syndromes: Management of STEMI




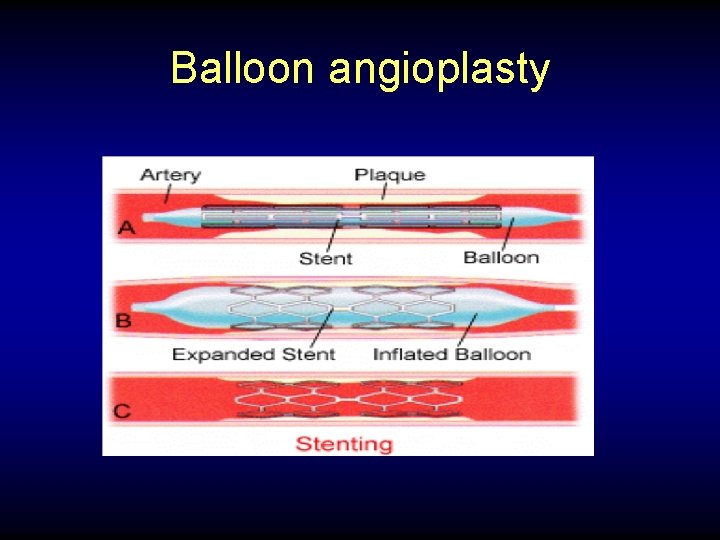
Balloon angioplasty

Stents

Acute Coronary Syndromes: Management of UA/NSTEMI

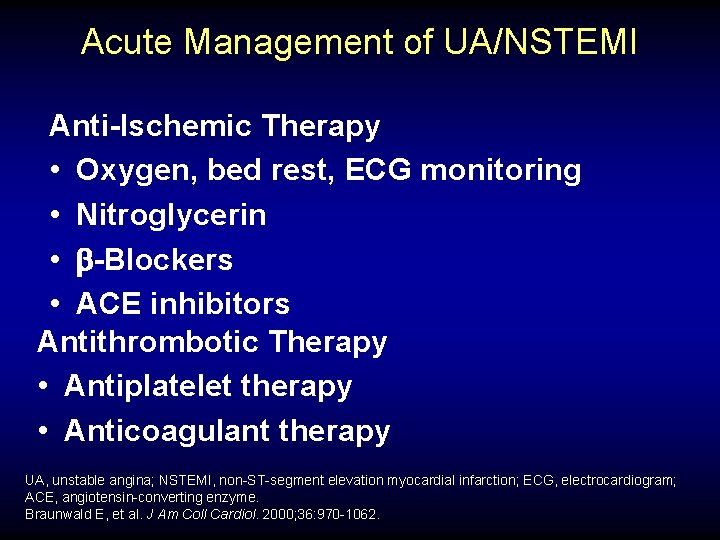
Acute Management of UA/NSTEMI Anti-Ischemic Therapy • Oxygen, bed rest, ECG monitoring • Nitroglycerin • -Blockers • ACE inhibitors Antithrombotic Therapy • Antiplatelet therapy • Anticoagulant therapy UA, unstable angina; NSTEMI, non-ST-segment elevation myocardial infarction; ECG, electrocardiogram; ACE, angiotensin-converting enzyme. Braunwald E, et al. J Am Coll Cardiol. 2000; 36: 970 -1062.

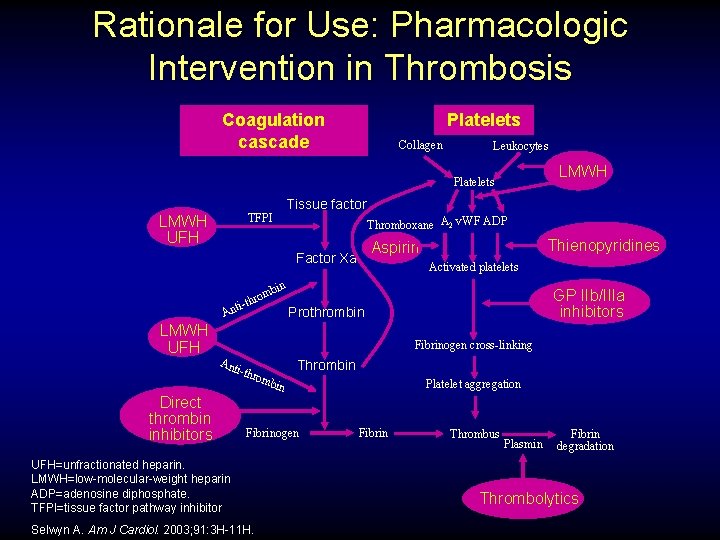
Rationale for Use: Pharmacologic Intervention in Thrombosis Coagulation cascade Platelets Collagen Leukocytes LMWH Platelets Tissue factor TFPI LMWH UFH Thromboxane A 2 v. WF ADP Thienopyridines Aspirin Factor Xa Activated platelets bin rom ti-th An LMWH UFH GP IIb/IIIa inhibitors Prothrombin Fibrinogen cross-linking Ant i-th Direct thrombin inhibitors Thrombin rom bin Fibrinogen UFH=unfractionated heparin. LMWH=low-molecular-weight heparin ADP=adenosine diphosphate. TFPI=tissue factor pathway inhibitor Selwyn A. Am J Cardiol. 2003; 91: 3 H-11 H. Platelet aggregation Fibrin Thrombus Plasmin Fibrin degradation Thrombolytics


Efficacy of Aspirin Doses on Vascular Events in High Risk Patients Aspirin Dose # Trials OR* (%) 500– 1500 mg 34 19 160– 325 mg 19 26 75– 150 mg 12 32 <75 mg Any aspirin 3 65 13 23 0 Odds Ratio 0. 5 Anti - platelet Better *Odds reduction. 1. 0 1. 5 2. 0 Anti - platelet Worse Treatment effect P < 0. 0001. Adapted with permission from the BMJ Publishing Group. Anti-thrombotic Trialists’ Collaboration. BMJ. 2002; 324: 71 -86.

Comparison of Heparin + ASA vs ASA Alone B Theroux B RISC B Cohen 1990 B ATACS B Summary Relative Risk 0. 67 (0. 44 -0. 1. 02) 0. 1 Heparin + ASA 55/698=7. 9% B Holdright Gurfinkel B 1 RR: Death/MI 10 ASA Alone 68/655=10. 4% ASA, acetylsalicylic acid; RISC, Research on In. Stability in Coronary artery disease; ATACS, Antithrombotic Therapy in Acute Company Syndromes; RR, relative risk; MI, myocardial infarction. Oler A, et al. JAMA. 1996; 276: 811 -815. (with permission)


TIMI IIB/ESSENCE Metanalysis: Enoxaparin vs Unfractionated Heparin Death or MI UFH (%) ENOX (%) 2 1. 8 1. 4 0. 80 (0. 55 -1. 16) 20 . 24 8 5. 3 4. 1 0. 77(0. 62 -0. 95) 23 . 02 14 6. 5 5. 2 0. 79 (0. 65 -0. 96) 21 . 02 43 8. 6 7. 1 0. 82 (0. 69 -0. 97) 18 . 02 0. 5 Favors ENOX OR (95 CI) % Day 1 OR P 2 Favors UFH TIMI, Thrombosis in Myocardial Infarction; ESSENCE, Efficacy and Safety of Subcutaneous Enozapam in Non–Q-Wave Coronary Events; UHF, unfractionated heparin; ENOX, enoxaparin; MI, myocardial infarction; OR, odds ratio. Antman EM, et al. Circulation. 1999; 100: 1602 -1608. (with permission)

LMWH Limitations • Indirect inhibition: dependent on antithrombin • Inability to inhibit clot-bound thrombin • Catheterization lab: slower onset, longer half-life, and with enoxaparin, no standard test to measure levels/effect • CABG: concerns regarding the long half-life • Monitoring for Factor Xa: possible, but what is therapeutic target, increased time, expense? • Not all LMWHs are the same CABG=coronary artery bypass graft.

CURE: Primary Endpoint: MI/Stroke/CV Death Cumulative Hazard Rate 0. 14 Placebo + Aspirin* 0. 12 20% Relative-risk Reduction 0. 10 0. 08 P<0. 001 N=12, 562 Clopidogrel + Aspirin* 0. 06 0. 04 0. 02 0. 00 0 3 6 9 Months of Follow-Up *Other standard therapies were used as appropriate. CV=cardiovascular. CURE Trial Investigators. N Engl J Med. 2001; 345: 494 -502. 12

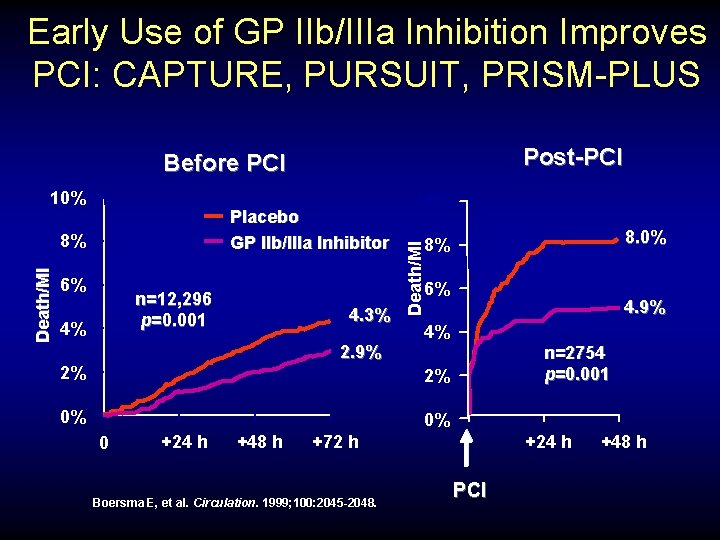
Early Use of GP IIb/IIIa Inhibition Improves PCI: CAPTURE, PURSUIT, PRISM-PLUS Post-PCI Before PCI Placebo GP IIb/IIIa Inhibitor Death/MI 8% 6% 8. 0% 8% 6% n=12, 296 p=0. 001 4% 10% Death/MI 10% 4. 3% 2. 9% 2% 4. 9% 4% n=2754 p=0. 001 2% 0% 0% 0 +24 h +48 h +24 h +72 h Boersma E, et al. Circulation. 1999; 100: 2045 -2048. PCI +48 h

In-hospital Mortality is Lower With Early GP IIb/IIIa Inhibitor Use (within 24 hrs) † ∆ 42% p<0. 0001 Includes patients who received late GP IIb/IIIa inhibitor (> 24 hrs) therapy. † Unadjusted for risk.

TIMI Risk Score for UA/NSTEMI: 7 Independent Predictors – Aged ≥ 65 years – ≥ 3 CAD risk factors – Prior CAD (stenosis >50%) – Aspirin in last 7 days – >2 anginal events in ≤ 24 hours – ST deviation – Elevated cardiac markers (CK-MB or troponin) TIMI, thrombosis in myocardial infarction; UA, unstable angina; NSTEMI, non–ST-segment elevation myocardial infarction; CAD, coronary artery disease. Antman EM, et al. JAMA. 2000; 284: 835 -842.

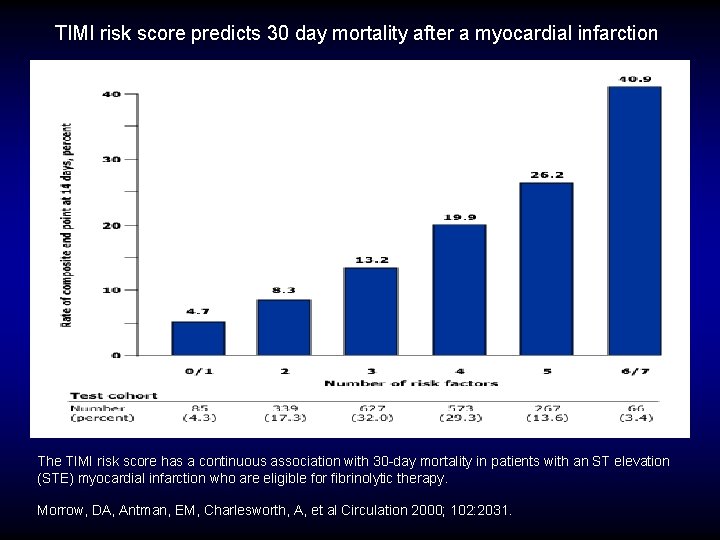
TIMI risk score predicts 30 day mortality after a myocardial infarction The TIMI risk score has a continuous association with 30 -day mortality in patients with an ST elevation (STE) myocardial infarction who are eligible for fibrinolytic therapy. Morrow, DA, Antman, EM, Charlesworth, A, et al Circulation 2000; 102: 2031.

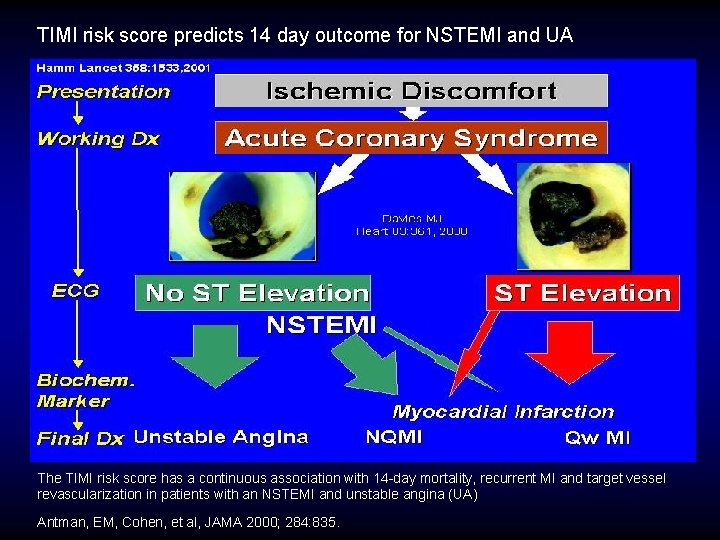
TIMI risk score predicts 14 day outcome for NSTEMI and UA The TIMI risk score has a continuous association with 14 -day mortality, recurrent MI and target vessel revascularization in patients with an NSTEMI and unstable angina (UA) Antman, EM, Cohen, et al, JAMA 2000; 284: 835.

STRIVE TM

STRIVE TM

STRIVE TM

Chest Pain Classification/Triage Class III Class IV Class “x”

Class I • STEMI Chest pain with STE or new LBBB • Acute revascularization followed by CICU admission

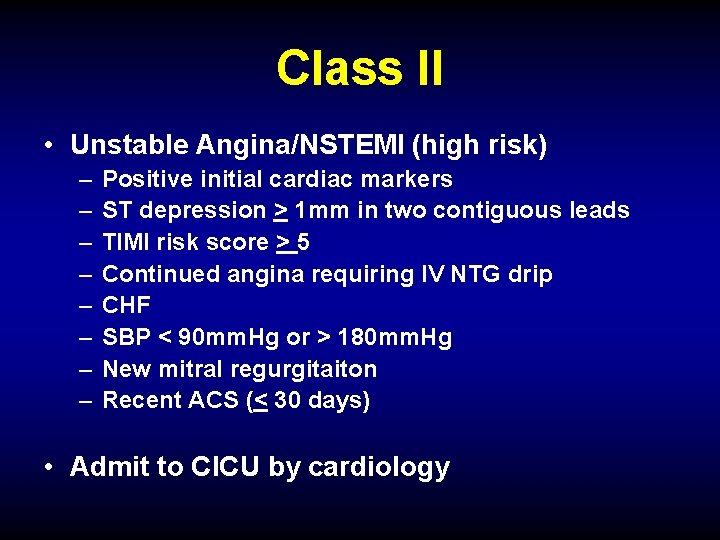
Class II • Unstable Angina/NSTEMI (high risk) – – – – Positive initial cardiac markers ST depression > 1 mm in two contiguous leads TIMI risk score > 5 Continued angina requiring IV NTG drip CHF SBP < 90 mm. Hg or > 180 mm. Hg New mitral regurgitaiton Recent ACS (< 30 days) • Admit to CICU by cardiology

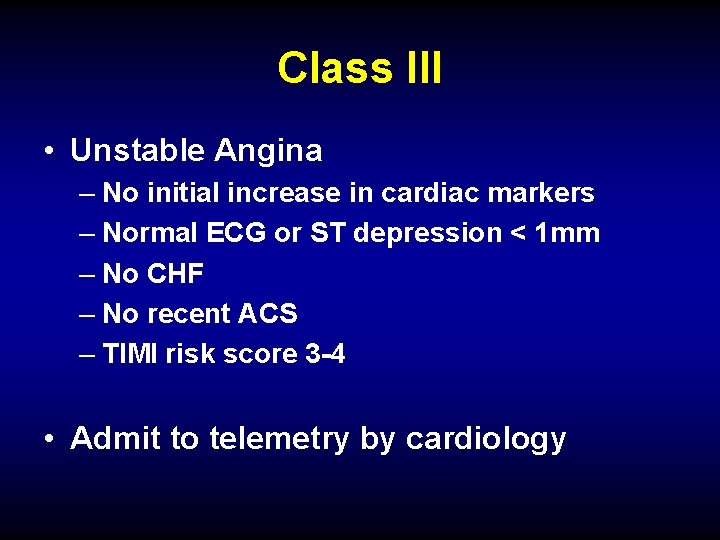
Class III • Unstable Angina – No initial increase in cardiac markers – Normal ECG or ST depression < 1 mm – No CHF – No recent ACS – TIMI risk score 3 -4 • Admit to telemetry by cardiology

Class IV • Unstable Angina with normal ECG – No initial increase in cardiac markers – No history of CAD – TIMI risk score < 3 – No ongoing or recurring pain • Admit to Chest Pain Service or Chest Pain Center in ER

Class V • Non-cardiac Chest Pain • Workup in ER with appropriate referral

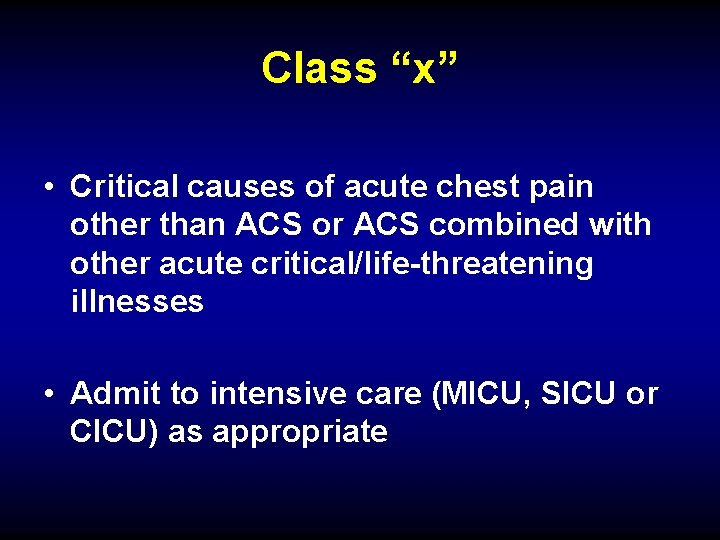
Class “x” • Critical causes of acute chest pain other than ACS or ACS combined with other acute critical/life-threatening illnesses • Admit to intensive care (MICU, SICU or CICU) as appropriate

Chest pain algorithm
 Dr saravanan kuppuswamy
Dr saravanan kuppuswamy Coronary artery disease
Coronary artery disease Coronary artery disease pathophysiology
Coronary artery disease pathophysiology Good morning blood
Good morning blood Right atrioventricular valve
Right atrioventricular valve Coronary heart disease
Coronary heart disease Branches of sma
Branches of sma Modified kuppuswamy
Modified kuppuswamy Uday pareek scale
Uday pareek scale Bharathi viswanathan
Bharathi viswanathan Pericardiu
Pericardiu Layers of the heart
Layers of the heart Coronary personality
Coronary personality Aortic semi lunar valve
Aortic semi lunar valve Ali sepahdari
Ali sepahdari Cardiac plexus
Cardiac plexus What is high quality cpr
What is high quality cpr Anterior aortic sinus
Anterior aortic sinus Dipyrimadole
Dipyrimadole Coronary sulcus
Coronary sulcus Global registry of acute coronary events
Global registry of acute coronary events Chronic coronary syndrome
Chronic coronary syndrome Pk papyrus covered coronary stent system
Pk papyrus covered coronary stent system Flow of coronary circulation
Flow of coronary circulation Acute coronary syndrome
Acute coronary syndrome Unlocking of knee joint
Unlocking of knee joint Coronary blood flow
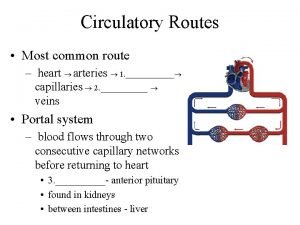
Coronary blood flow Coronary circulatory routes
Coronary circulatory routes Mesa coronary calcium score
Mesa coronary calcium score Heart disease
Heart disease Crash course heart
Crash course heart Coronary circulation animation
Coronary circulation animation Coronary circulation of heart
Coronary circulation of heart Mesa coronary calcium score
Mesa coronary calcium score Coronary angiography equipment
Coronary angiography equipment Coronary calcium score guidelines
Coronary calcium score guidelines Qfr coronary
Qfr coronary Pulmonary semilunar valve
Pulmonary semilunar valve Pk papyrus stent
Pk papyrus stent Sinusoid anatomy
Sinusoid anatomy Dr eter
Dr eter Nursing care for gastrointestinal disorders
Nursing care for gastrointestinal disorders Hirschsprung disease nursing management
Hirschsprung disease nursing management Kate lorig stanford
Kate lorig stanford Disease management association of america
Disease management association of america Developed by ed
Developed by ed Nursing management of cirrhosis of liver
Nursing management of cirrhosis of liver Disease management roi
Disease management roi Deka vitaminok
Deka vitaminok Disease management roi
Disease management roi Scientific management
Scientific management Top management middle management first line management
Top management middle management first line management Middle level management
Middle level management Posterior tibial artery
Posterior tibial artery Palmar arch artery
Palmar arch artery Ica eca anatomy
Ica eca anatomy Superior thyroid artery is a branch of
Superior thyroid artery is a branch of Figure 15-3 is a diagram of the nephron
Figure 15-3 is a diagram of the nephron Branches of radial artery
Branches of radial artery Canal de hunter
Canal de hunter Fascia of the neck
Fascia of the neck Branches of popliteal artery
Branches of popliteal artery Right marginal artery
Right marginal artery Triangular interval
Triangular interval Posterior axilla
Posterior axilla Structure of an artery
Structure of an artery Tubule
Tubule Right and left gastroepiploic arteries
Right and left gastroepiploic arteries Sensory innervation sole of foot
Sensory innervation sole of foot Pterion skull
Pterion skull Root of neck
Root of neck Persistent stapedial artery
Persistent stapedial artery Left inferior epigastric artery
Left inferior epigastric artery Posterolateral abdominal wall
Posterolateral abdominal wall Inferior border of the lung
Inferior border of the lung Internal iliac artery mnemonic
Internal iliac artery mnemonic Maxillary artery branches
Maxillary artery branches Sinusoidal capillaries
Sinusoidal capillaries Left and right gastric artery anastomosis
Left and right gastric artery anastomosis Lenticulate striate artery
Lenticulate striate artery Tunica intima
Tunica intima Parotid gland fascia
Parotid gland fascia Collateral branch of intercostal nerve
Collateral branch of intercostal nerve Testis artery
Testis artery Bursa coracobrachialis
Bursa coracobrachialis Tibial pulse location
Tibial pulse location Femoral ring
Femoral ring Inferior labial nerve
Inferior labial nerve Rvsp on echo
Rvsp on echo External carotid
External carotid Femoral canal and femoral sheath
Femoral canal and femoral sheath Deep artery of the arm
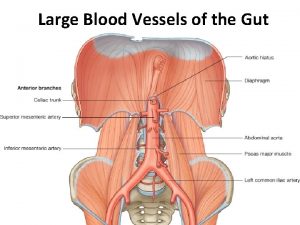
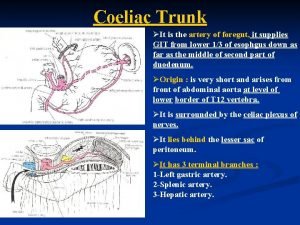
Deep artery of the arm Branches of celiac trunk
Branches of celiac trunk Types of venules
Types of venules Blood supply of pons
Blood supply of pons Mhyeo2fwsps -site:youtube.com
Mhyeo2fwsps -site:youtube.com Artery vein capillary structure
Artery vein capillary structure Pulmonary artery and aorta
Pulmonary artery and aorta 5 d's and 3 n's
5 d's and 3 n's Brachiocephalic vein cat
Brachiocephalic vein cat