Management and Quality Evaluation of Influenza Surveillance Network





















- Slides: 35

Management and Quality Evaluation of Influenza Surveillance Network in China Dr. Wang Dayan WHO Collaborating Center for Reference and Research on Influenza National Influenza Center of China National Institute for Viral Disease control and Prevention China CDC 2011 -6 -10, Vientiane , Lao’s CHINESE CENTER FOR DISEASE CONTROL AND PREVENTION

Content Ø Brief introduction of Chinese NISN and Responsibility of NISN Ø Management and Quality Evaluation of NISN Ø Changes CHINESE CENTER FOR DISEASE CONTROL AND PREVENTION

Part 1: Introduction of Chinese NISN and Responsibility of NISN CHINESE CENTER FOR DISEASE CONTROL AND PREVENTION

History of Chinese NIC and National Influenza Surveillance Network (NISN) Started influenza surveillance 1952 Chinese NIC jointed GISN 1957 1981 Established Chinese National Influenza Center CHINESE CENTER FOR DISEASE CONTROL AND PREVENTION

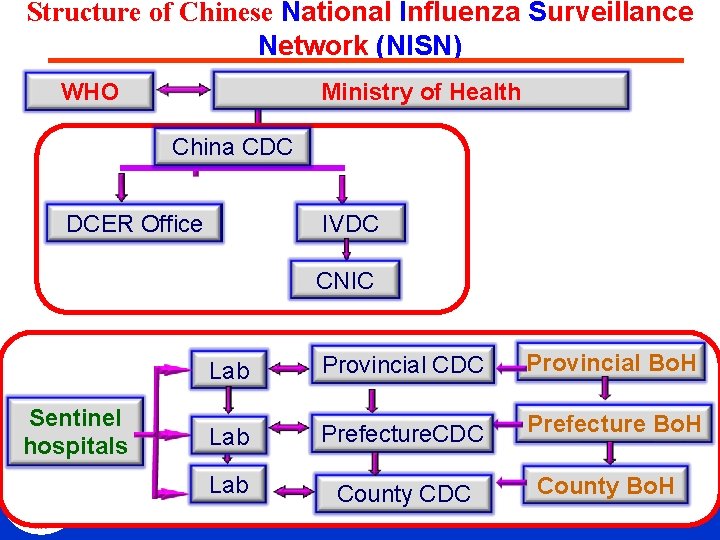
Structure of Chinese National Influenza Surveillance Network (NISN) Ministry of Health WHO China CDC DCER Office IVDC CNIC Sentinel hospitals Lab Provincial CDC Provincial Bo. H Lab Prefecture. CDC Prefecture Bo. H Lab County CDC County Bo. H CHINESE CENTER FOR DISEASE CONTROL AND PREVENTION

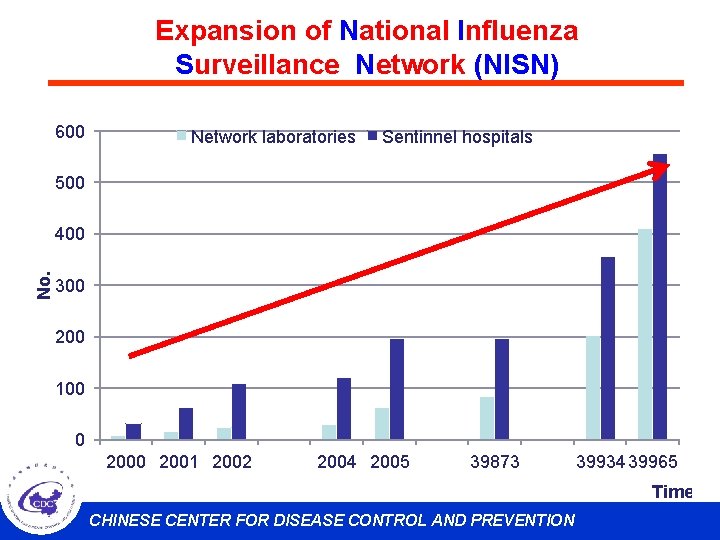
Expansion of National Influenza Surveillance Network (NISN) 600 Network laboratories Sentinnel hospitals 500 No. 400 300 200 100 0 2001 2002 2004 2005 39873 39934 39965 Time CHINESE CENTER FOR DISEASE CONTROL AND PREVENTION

Expansion of National Influenza Surveillance Network(NISN) 63 network labs 197 sentinel hospitals Before April, 2009 411 network labs 556 sentinel hospitals June, 2009 CHINESE CENTER FOR DISEASE CONTROL AND PREVENTION

Surveillance Objectives l To monitor influenza activity in mainland China. l To Determine what influenza viruses are circulating. l To detect changes in antigenic, genetic characteristics, and provide evidence for recommending circulating strain and vaccine strain l To monitor the novel influenza virus for the response to influenza pandemic. CHINESE CENTER FOR DISEASE CONTROL AND PREVENTION 8

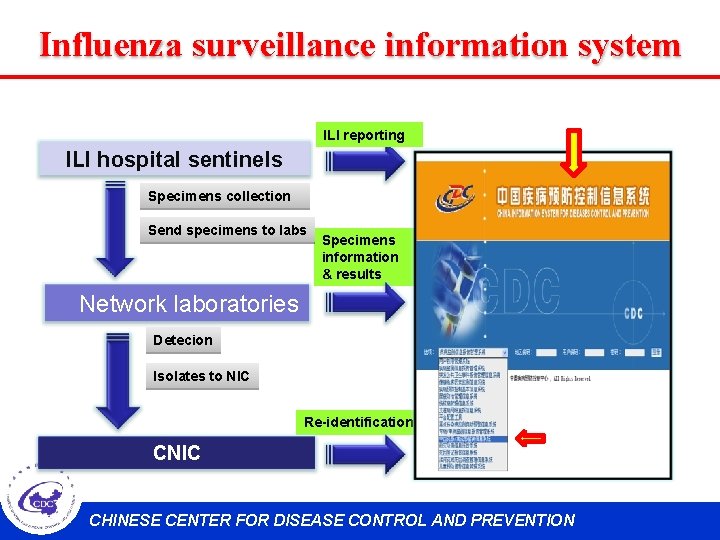
Influenza surveillance information system ILI reporting ILI hospital sentinels Specimens collection Send specimens to labs Specimens information & results Network laboratories Detecion Isolates to NIC Re-identification CNIC CHINESE CENTER FOR DISEASE CONTROL AND PREVENTION

Real-time Database(1) ILI report to Influenza Surveillance information system No. of ILI by age group CHINESE CENTER FOR DISEASE CONTROL AND PREVENTION Total no. of patients

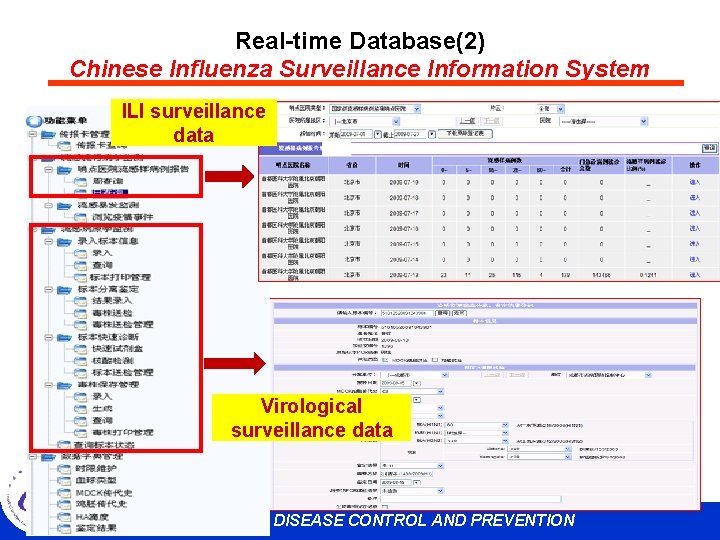
Real-time Database(2) Chinese Influenza Surveillance Information System ILI surveillance data Virological surveillance data CHINESE CENTER FOR DISEASE CONTROL AND PREVENTION

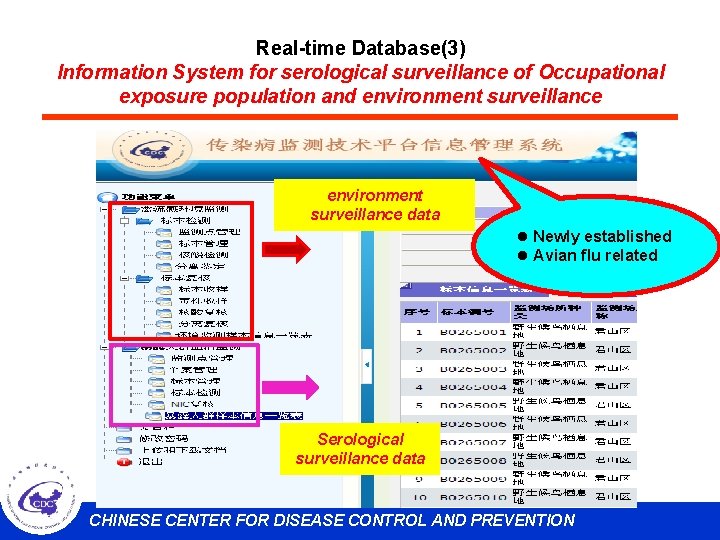
Real-time Database(3) Information System for serological surveillance of Occupational exposure population and environment surveillance data l Newly established l Avian flu related Serological surveillance data CHINESE CENTER FOR DISEASE CONTROL AND PREVENTION

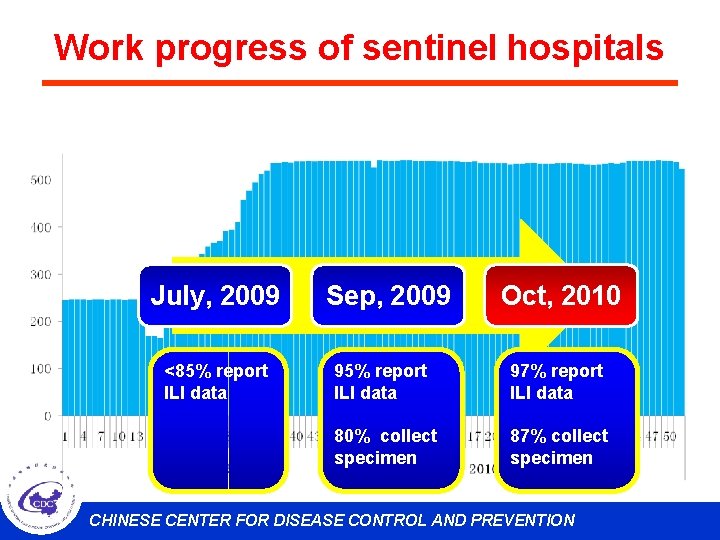
Work progress of sentinel hospitals July, 2009 <85% report ILI data Sep, 2009 Oct, 2010 95% report ILI data 97% report ILI data 80% collect specimen 87% collect specimen CHINESE CENTER FOR DISEASE CONTROL AND PREVENTION

Work progress of network labs CHINESE CENTER FOR DISEASE CONTROL AND PREVENTION

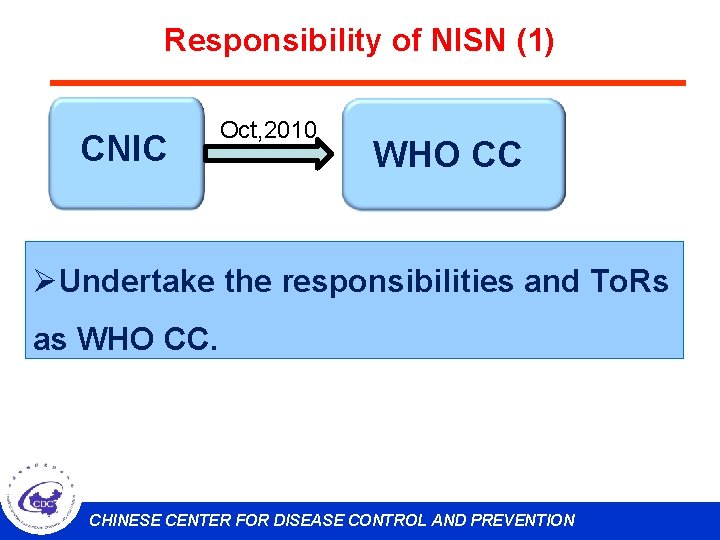
Responsibility of NISN (1) CNIC Oct, 2010 WHO CC ØUndertake the responsibilities and To. Rs as WHO CC. CHINESE CENTER FOR DISEASE CONTROL AND PREVENTION

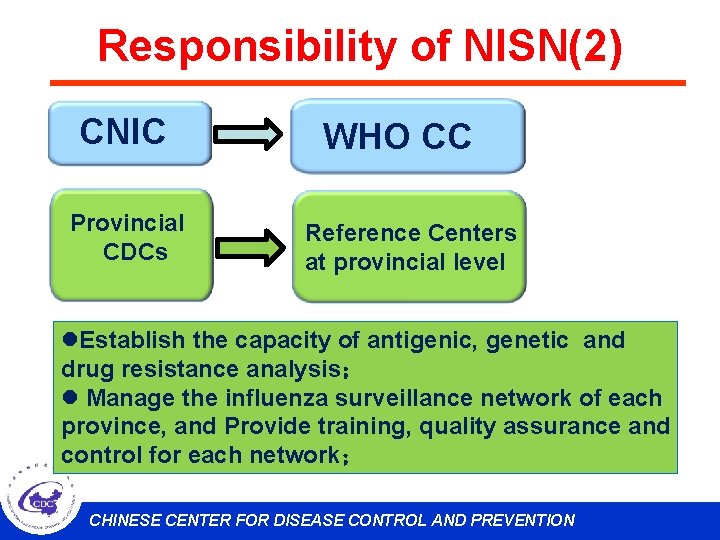
Responsibility of NISN(2) CNIC Provincial CDCs WHO CC Reference Centers at provincial level l. Establish the capacity of antigenic, genetic and drug resistance analysis; l Manage the influenza surveillance network of each province, and Provide training, quality assurance and control for each network; CHINESE CENTER FOR DISEASE CONTROL AND PREVENTION

Responsibility of NISN(3) CNIC Provincial CDCs Network Labs WHO CC Reference Centers at provincial level Labs with the capacity of virus isolation and nucleic test l. Establish the capacity of virus isolation and nucleic test; l. Organize, manage the hospital-based ILI surveillance CHINESE CENTER FOR DISEASE CONTROL AND PREVENTION

Part 2: Management and Quality Evaluation of NISN CHINESE CENTER FOR DISEASE CONTROL AND PREVENTION

Routine technical support to NISN Provide standard reference reagents Training workshops: year round National influenza surveillance guideline &Technical manual CHINESE CENTER FOR DISEASE CONTROL AND PREVENTION

Providing reagents to NISN RT-PCR l. Reagents for HI test seasonal virus, serology test l. Primer/probe sets CHINESE CENTER FOR DISEASE CONTROL AND PREVENTION r. RT-PCR

Trainings to the NISN every year l. CNIC organized l. Provincial CDC organized l----Workshops, hands on training Sample collection virus isolation nucleic acid test CHINESE CENTER FOR DISEASE CONTROL AND PREVENTION

National influenza surveillance guideline &Technical manual April, 30, 2009 May, 11, 2009 May, 03, 2009 Sep, 3, 2010 Sep, 30, 2009 CHINESE CENTER FOR DISEASE CONTROL AND PREVENTION

Quality assessment to NISN Real time feed back of the test results of CNIC to NISN through the surveillance system External quality assessment panel for nucleic acid test for NISN every year Site supervision to network labs every year Quality assessment to NISN twice a year CHINESE CENTER FOR DISEASE CONTROL AND PREVENTION

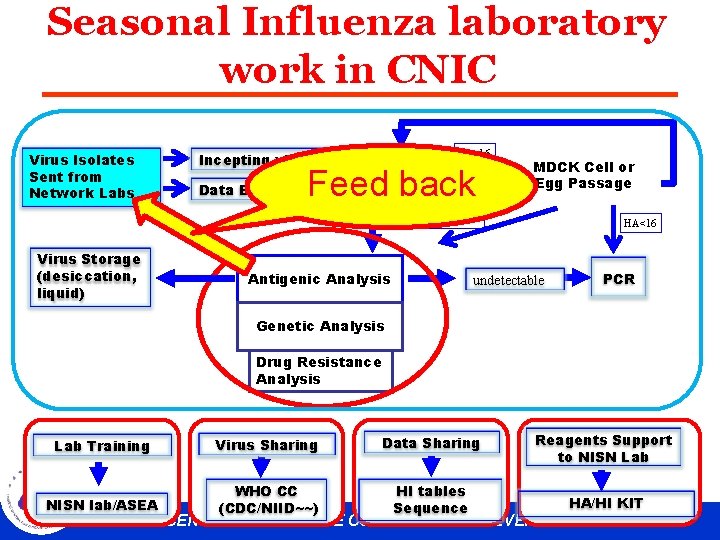
Seasonal Influenza laboratory work in CNIC Virus Isolates Sent from Network Labs HA<16 Incepting virus Data Entry Feed back HA MDCK Cell or Egg Passage HA≥ 16 Virus Storage (desiccation, liquid) Antigenic Analysis HA<16 PCR undetectable Genetic Analysis Drug Resistance Analysis Lab Training Virus Sharing Data Sharing Reagents Support to NISN Lab NISN lab/ASEA WHO CC (CDC/NIID~~) HI tables Sequence HA/HI KIT CHINESE CENTER FOR DISEASE CONTROL AND PREVENTION

Ferret Anti-sera H 3 N 2 HIN 1 H 1 N 1 pdm B-Vic BYam CHINESE CENTER FOR DISEASE CONTROL AND PREVENTION

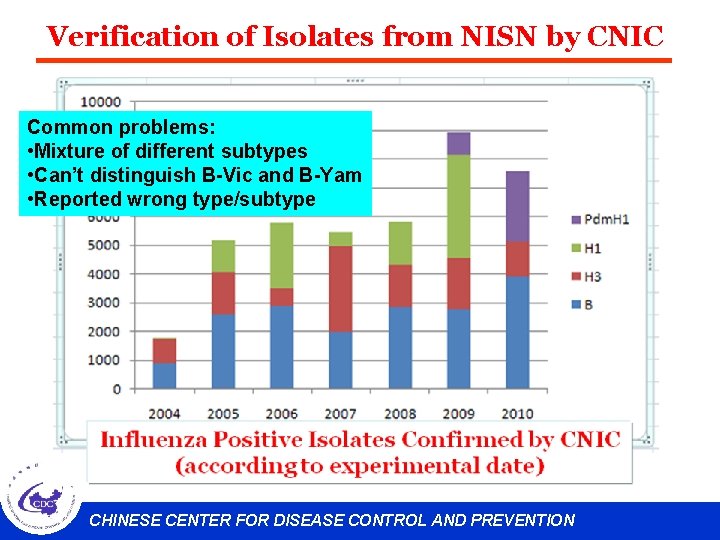
Verification of Isolates from NISN by CNIC Common problems: • Mixture of different subtypes • Can’t distinguish B-Vic and B-Yam • Reported wrong type/subtype CHINESE CENTER FOR DISEASE CONTROL AND PREVENTION

QAP for nucleic acid test Year No. of laboratories participated 2007 30 provincial network laboratories 2008 30 provincial network laboratories 31 local network laboratories 2009 411 labs 2010 411 labs ØPanel: 10 samples: β-propiolactone inactivated egg isolates ØTest the stability: frozen/thaw, RT ØConcentration: ØCross detection of different subtypes CHINESE CENTER FOR DISEASE CONTROL AND PREVENTION

QAP for nucleic acid test CHINESE CENTER FOR DISEASE CONTROL AND PREVENTION

Supervision CHINESE CENTER FOR DISEASE CONTROL AND PREVENTION

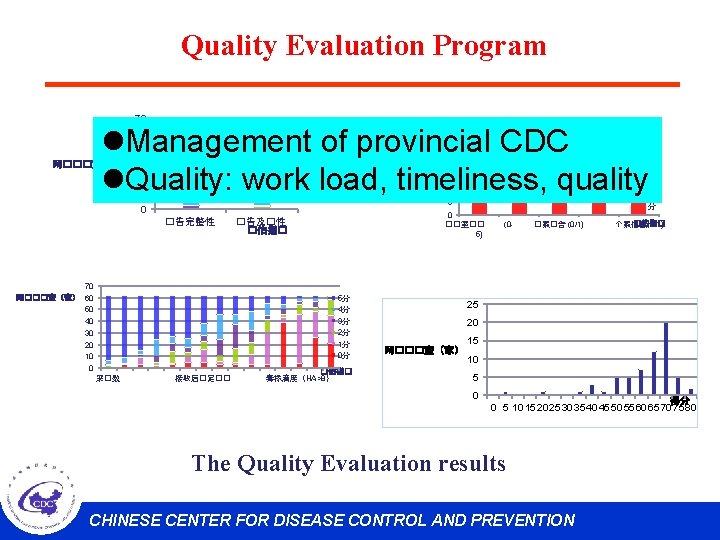
Quality Evaluation Program 70 60 50 40 网���室(家) 30 20 10 0 35 l. Management of provincial CDC l. Quality: work load, timeliness, quality 30 5分 4分 3分 2分 1分 25 10 5 �告完整性 70 网���室(家) 60 50 40 30 20 10 0 0 ��室�� 5) �告及�性 �估指� 5分 4分 3分 2分 1分 0分 采�数 5 分 4 分 3 分 20 省/市(家) 15 接收后�定�� �估指� 毒株滴度(HA>8) (0 - �案�告 (0/1) �估指� 个案信息(0/1) 25 20 网���室(家) 15 10 5 0 得分 0 5 10 15 20 25 30 35 40 45 50 55 60 65 70 75 80 The Quality Evaluation results CHINESE CENTER FOR DISEASE CONTROL AND PREVENTION

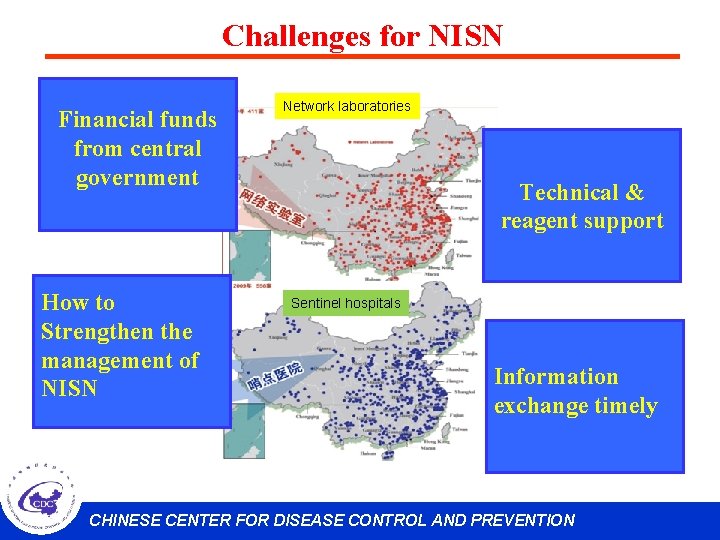
Challenges for NISN Financial funds from central government How to Strengthen the management of NISN Network laboratories Technical & reagent support Sentinel hospitals Information exchange timely CHINESE CENTER FOR DISEASE CONTROL AND PREVENTION

Conclusions and suggestions l Governmental coordination plays a key role l Scientific based decision-making is the fundamental for public health l Pandemic preparedness for H 5 N 1 & other subtypes are rewarded l International cooperation is essential for the world CHINESE CENTER FOR DISEASE CONTROL AND PREVENTION

Contact information for WHOCC China Director: Dr. Yuelong Shu WHO Collaborating Center for Reference and Research on Influenza, National Institute for Viral Disease Control and Prevention, China CDC. 155 Changbai Road, Changping District, 102206, Beijing, China. Tel: +8610 58900851, Fax: +8610 58900851, Email: whocc-china@cnic. org. cn, Website : http: // www. cnic. org. cn/eng/ CHINESE CENTER FOR DISEASE CONTROL AND PREVENTION

Acknowledgments • • WHO CDC WHOCCs CCDC/CNIC colleagues CHINESE CENTER FOR DISEASE CONTROL AND PREVENTION

Thank You! CHINESE CENTER FOR DISEASE CONTROL AND PREVENTION
 Piano di divisione delle staffe
Piano di divisione delle staffe Causative agent
Causative agent The great influenza rhetorical analysis
The great influenza rhetorical analysis Albert osterhaus
Albert osterhaus Influenza
Influenza Stomach flu vs influenza
Stomach flu vs influenza Is influenza a airborne disease
Is influenza a airborne disease Fibertel
Fibertel Influenza vaccine dosage chart 2019-2020
Influenza vaccine dosage chart 2019-2020 Influenza ww1
Influenza ww1 Influenza virus replication
Influenza virus replication Low pathogenic avian influenza
Low pathogenic avian influenza Contemporary
Contemporary Quality assurance vs quality control
Quality assurance vs quality control Concepts of quality
Concepts of quality Perceptual evaluation of speech quality
Perceptual evaluation of speech quality Fqem
Fqem Quality management in operations management
Quality management in operations management Operations management with total quality management book
Operations management with total quality management book Continuing analysis and surveillance system
Continuing analysis and surveillance system Reconnaissance and surveillance leaders course
Reconnaissance and surveillance leaders course Informant management
Informant management Regulatory agencies
Regulatory agencies Quality management pmp
Quality management pmp Quality metrics pmp
Quality metrics pmp Define quality assurance in nursing
Define quality assurance in nursing Compliance vs quality
Compliance vs quality Which one is jurans three role model
Which one is jurans three role model Crosby's fourteen steps to quality improvement
Crosby's fourteen steps to quality improvement What is tqm
What is tqm Network performance management system
Network performance management system Network accounting management
Network accounting management Accounting management in network management
Accounting management in network management Network management definition
Network management definition Datagram switching vs virtual circuit
Datagram switching vs virtual circuit Features of peer to peer network and client server network
Features of peer to peer network and client server network