Male Reproductive System Undergraduate Graduate Histology Lecture Series














- Slides: 141

Male Reproductive System Undergraduate – Graduate Histology Lecture Series Larry Johnson, Professor Veterinary Integrative Biosciences Texas A&M University College Station, TX 77843

Objectives To examine the testis (which produce spermatozoa), excretory ducts (which transport and mature spermatozoa), and accessory glands (whose secretions support the viability of spermatozoa) for characteristics and functions the male reproductive system. To learn what structures and hormonal regulation facilitate the male gonad to produce an exocrine secretion (the spermatozoon) and an endocrine secretion (testosterone).

Outline History Spermatozoon Spermatogenesis Sertoli and Leydig cells Hormonal control Epididymal and accessory glans characteristics and functions Fertile ejaculate

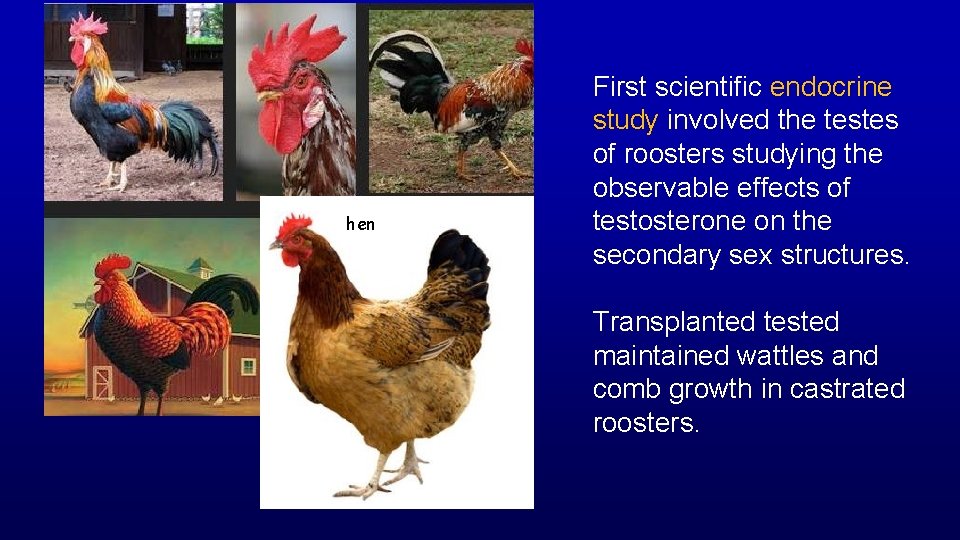
hen First scientific endocrine study involved the testes of roosters studying the observable effects of testosterone on the secondary sex structures. Transplanted tested maintained wattles and comb growth in castrated roosters.

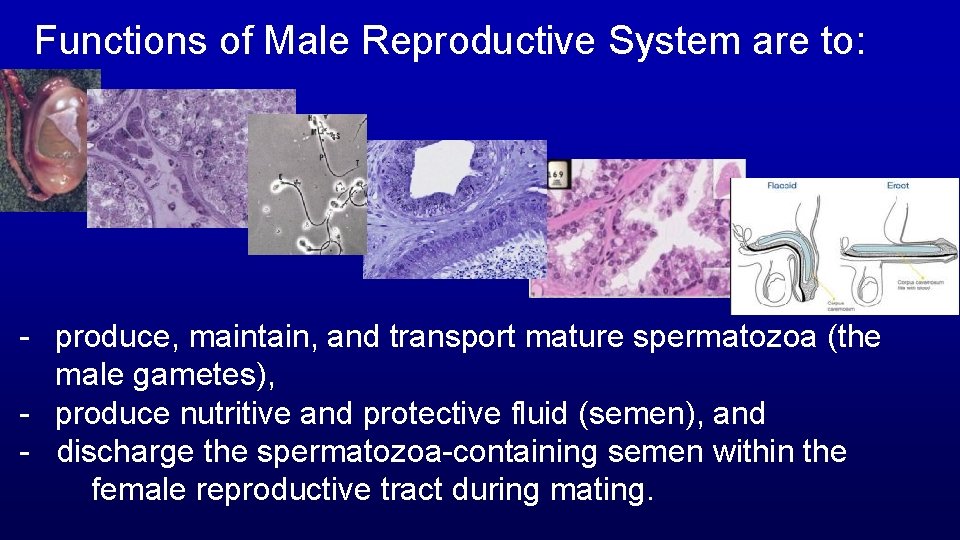
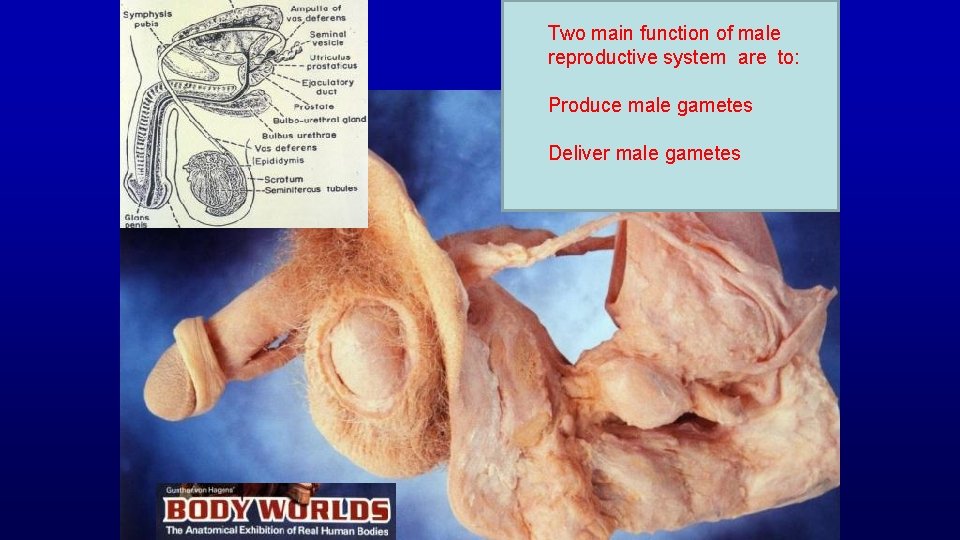
Functions of Male Reproductive System are to: - produce, maintain, and transport mature spermatozoa (the male gametes), - produce nutritive and protective fluid (semen), and - discharge the spermatozoa-containing semen within the female reproductive tract during mating.





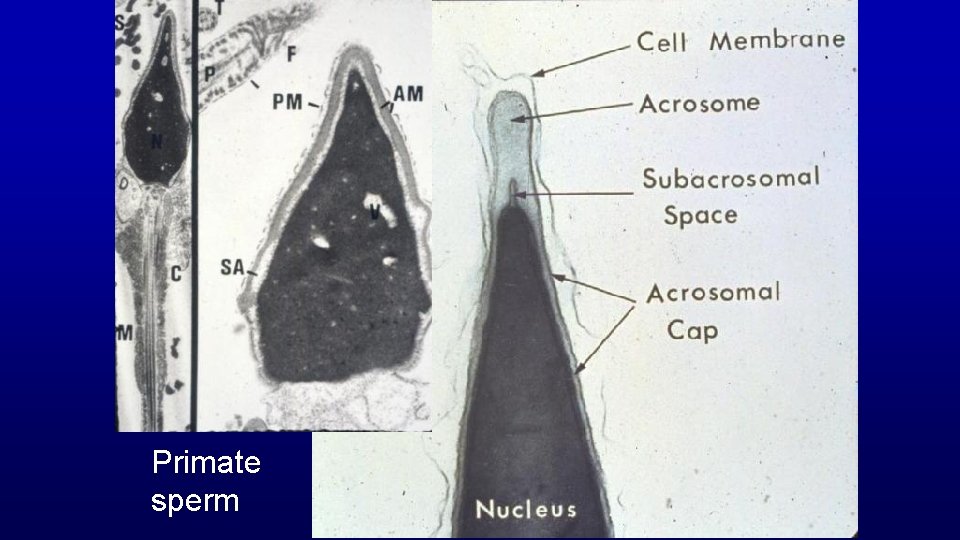
Primate sperm

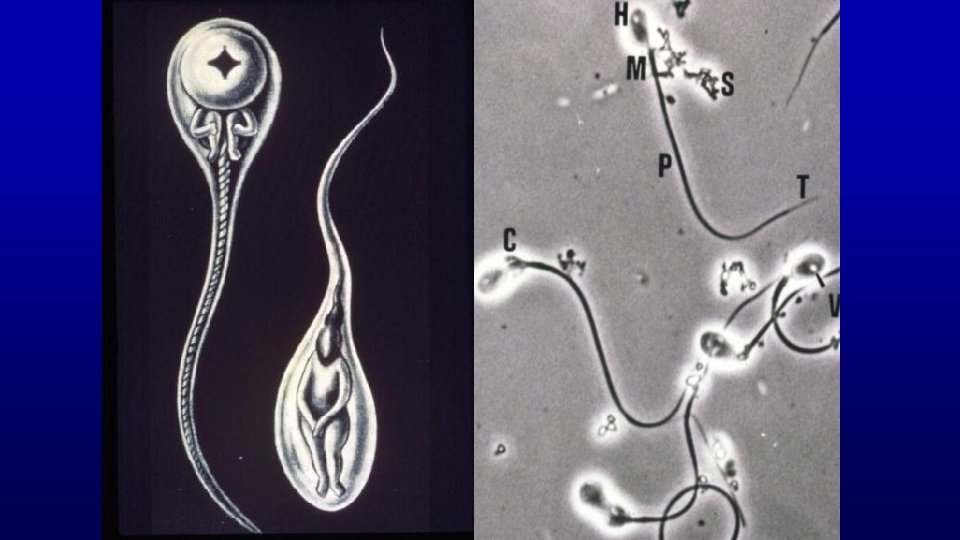
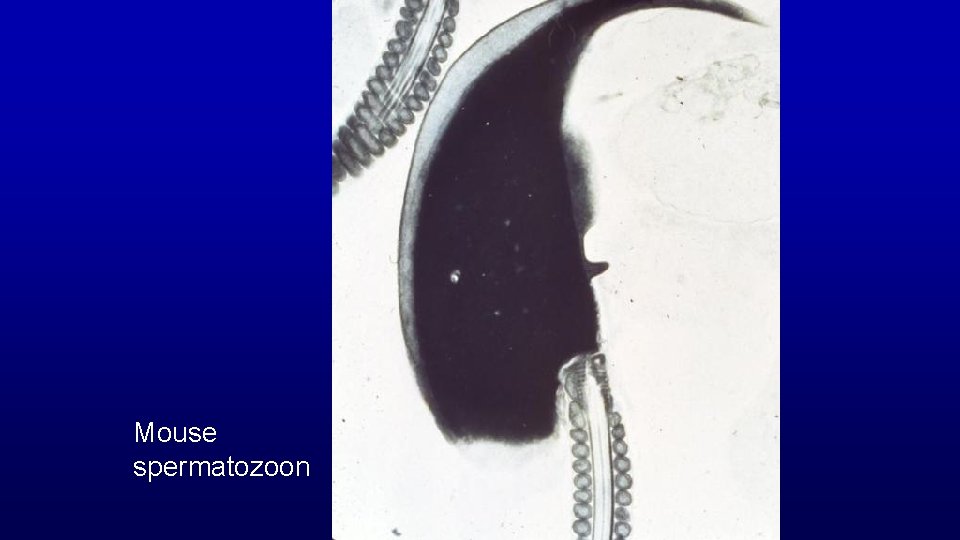
Mouse spermatozoon



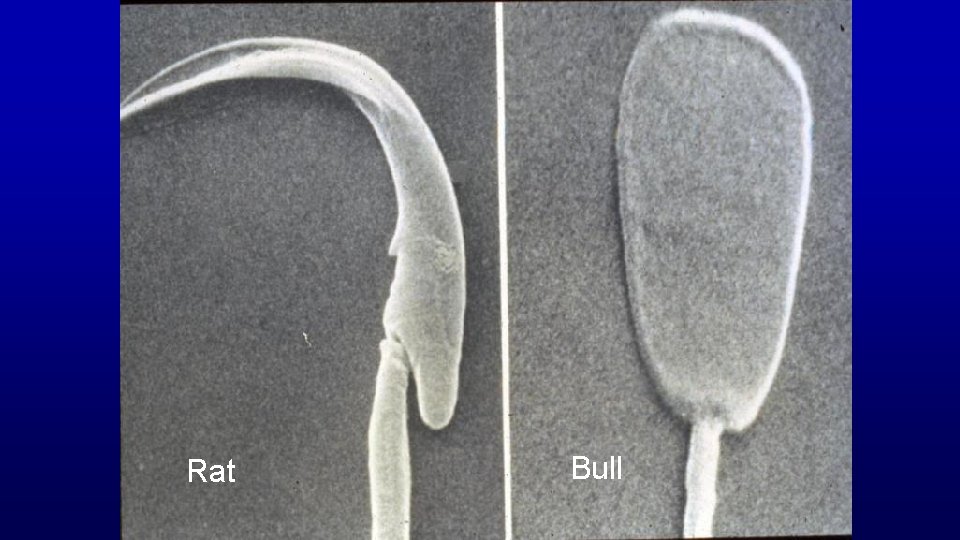
Rat Bull


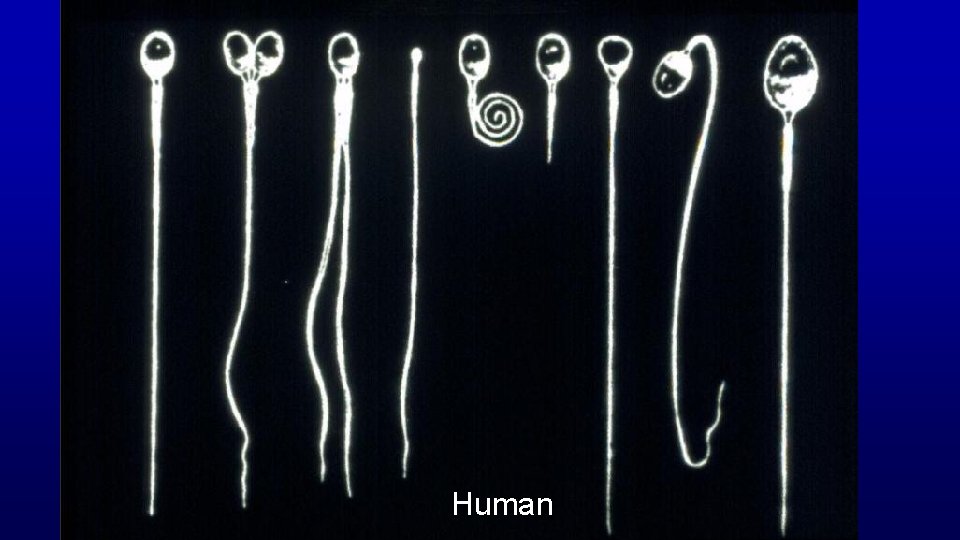
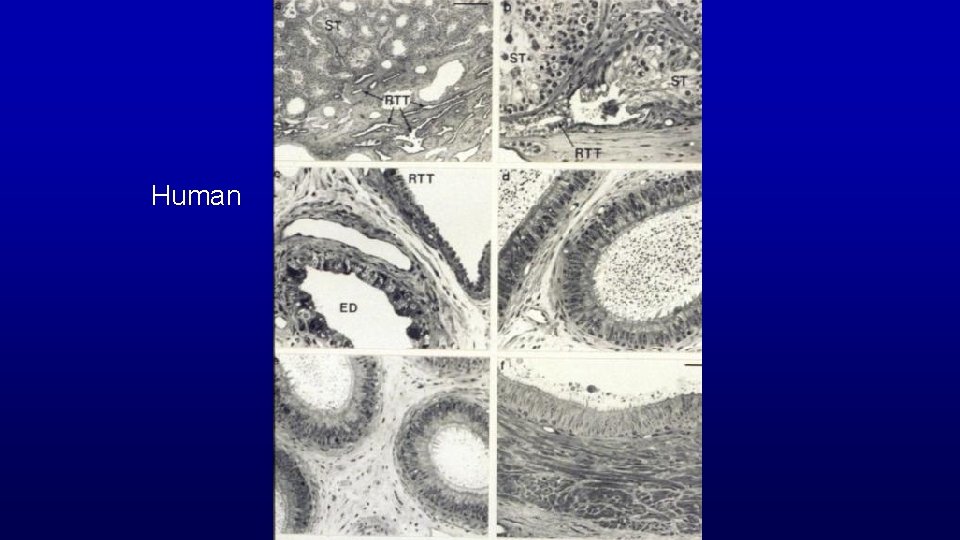
Human

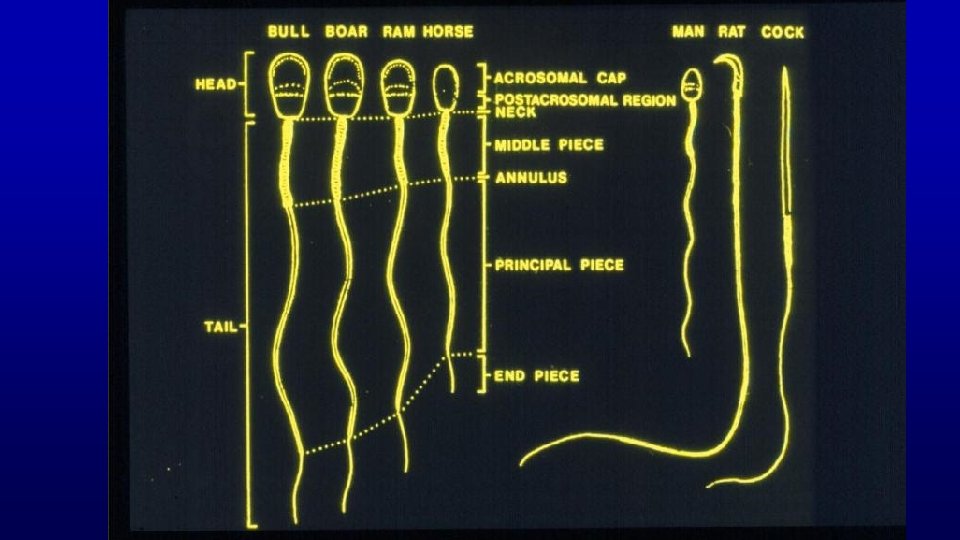
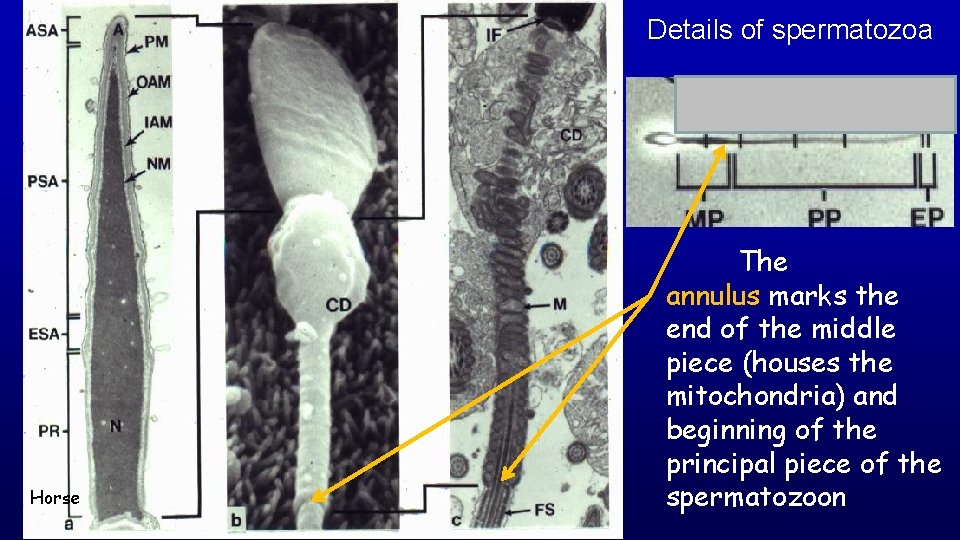
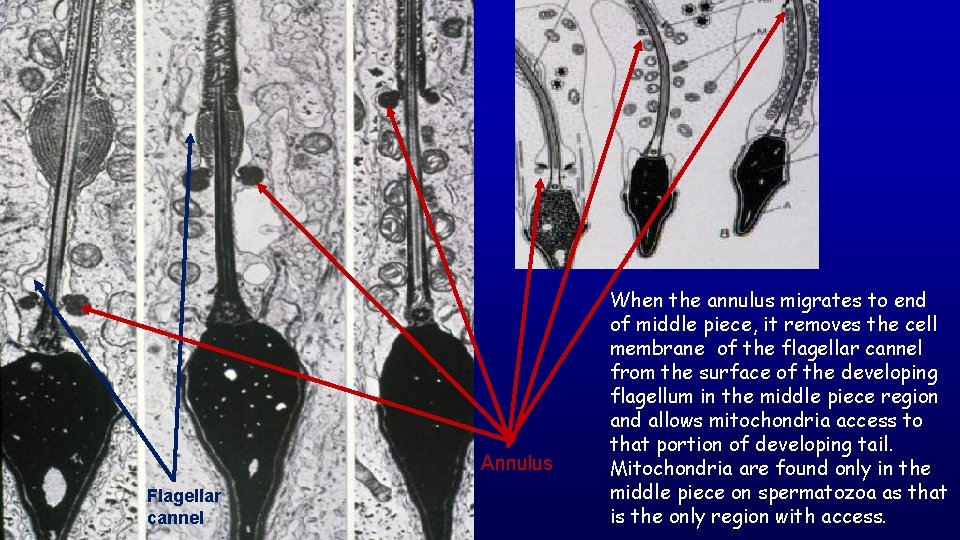
Details of spermatozoa Horse The annulus marks the end of the middle piece (houses the mitochondria) and beginning of the principal piece of the spermatozoon

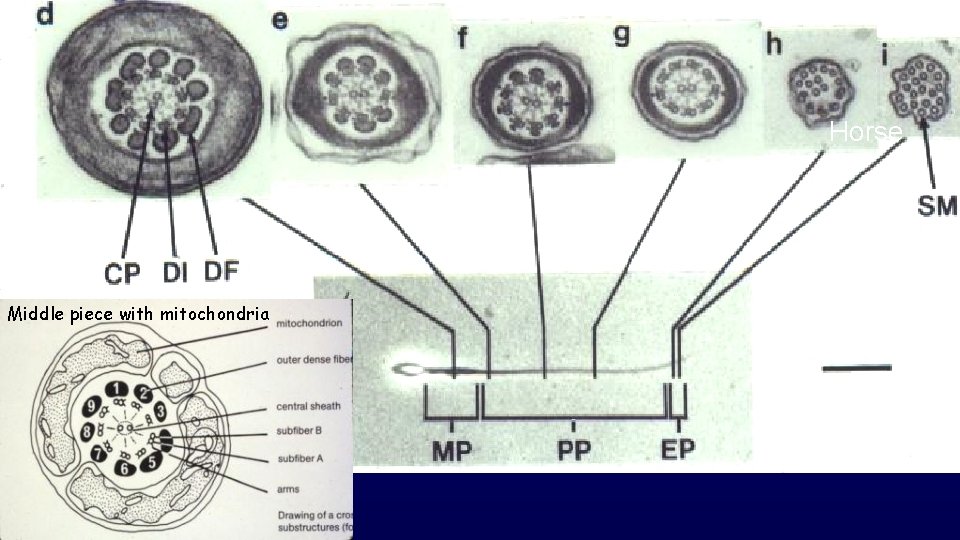
Horse Middle piece with mitochondria

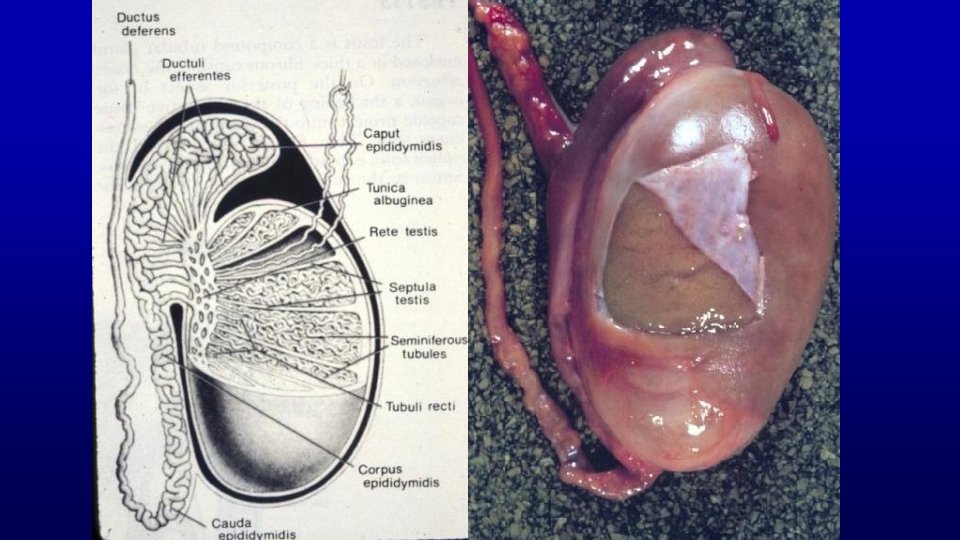
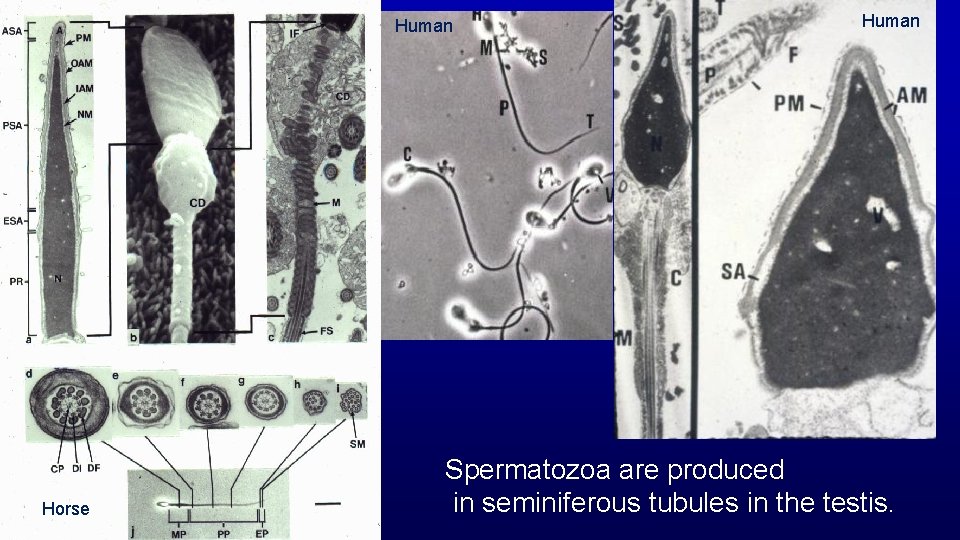
Human Horse Human Spermatozoa are produced in seminiferous tubules in the testis.

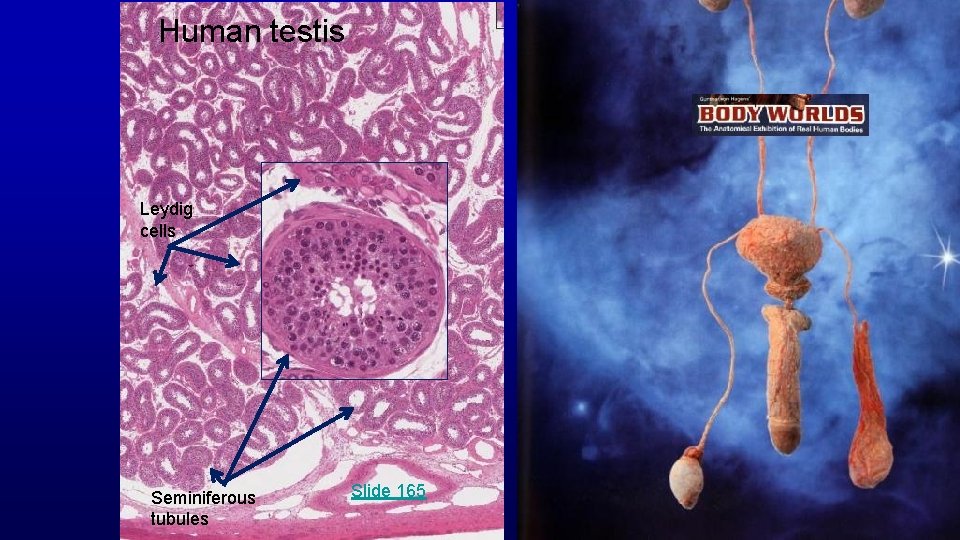
Human testis Leydig cells Seminiferous tubules Slide 165

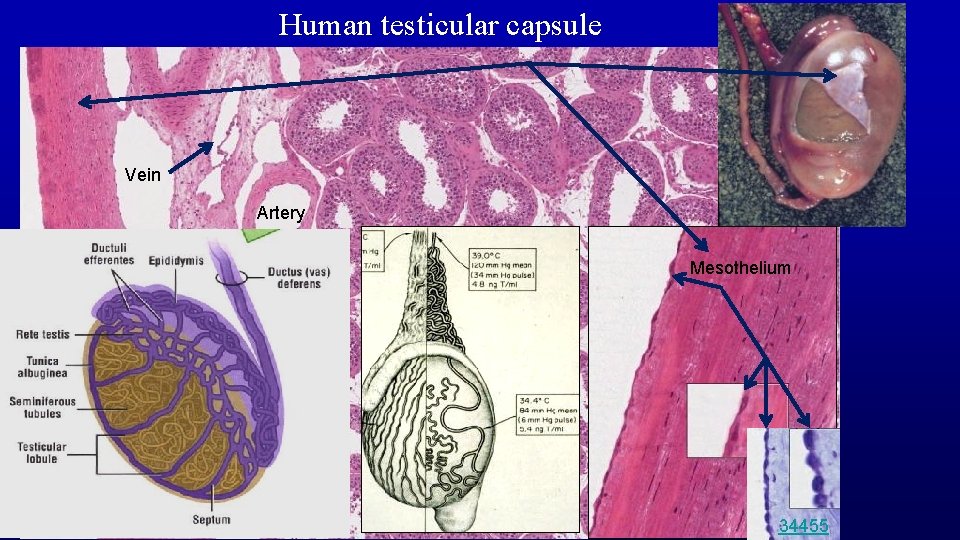
Human testicular capsule Vein Artery Mesothelium Slide 165 34455

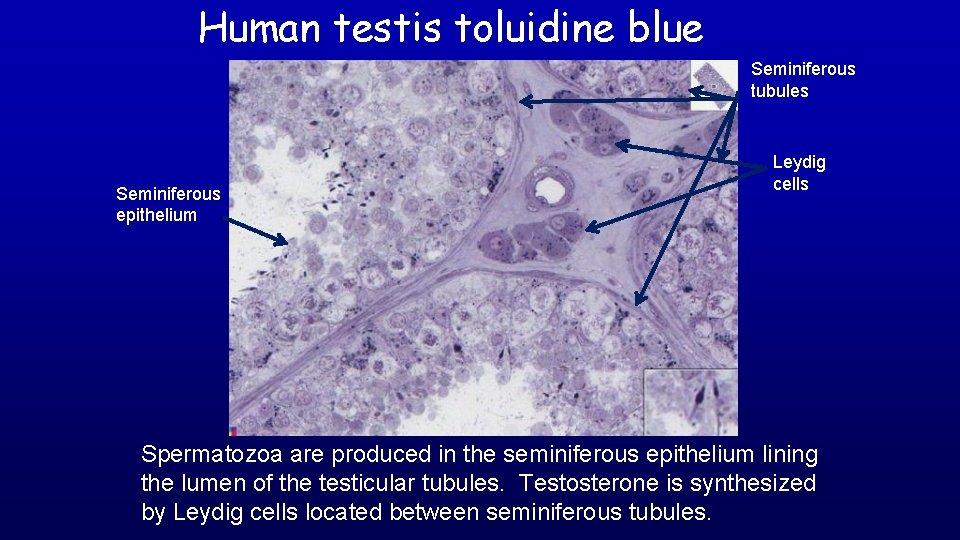
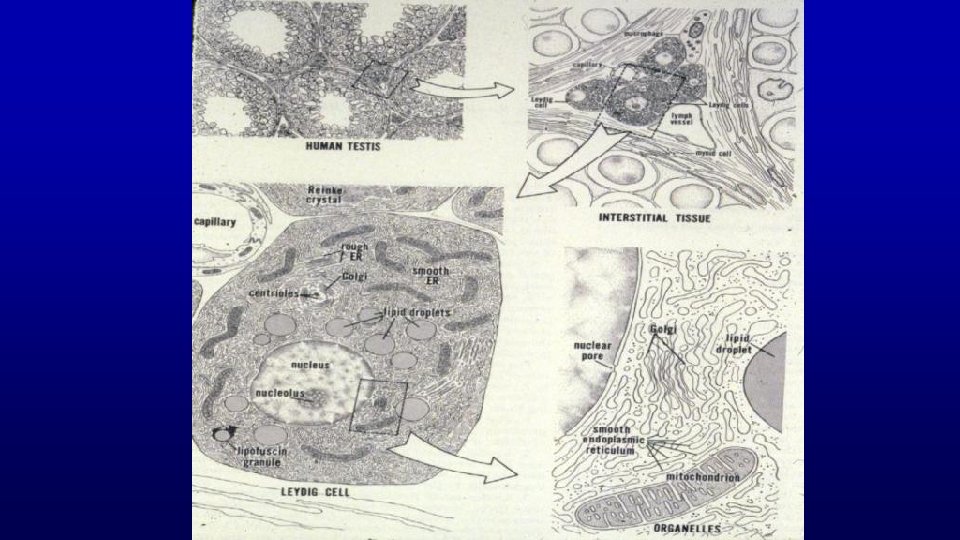
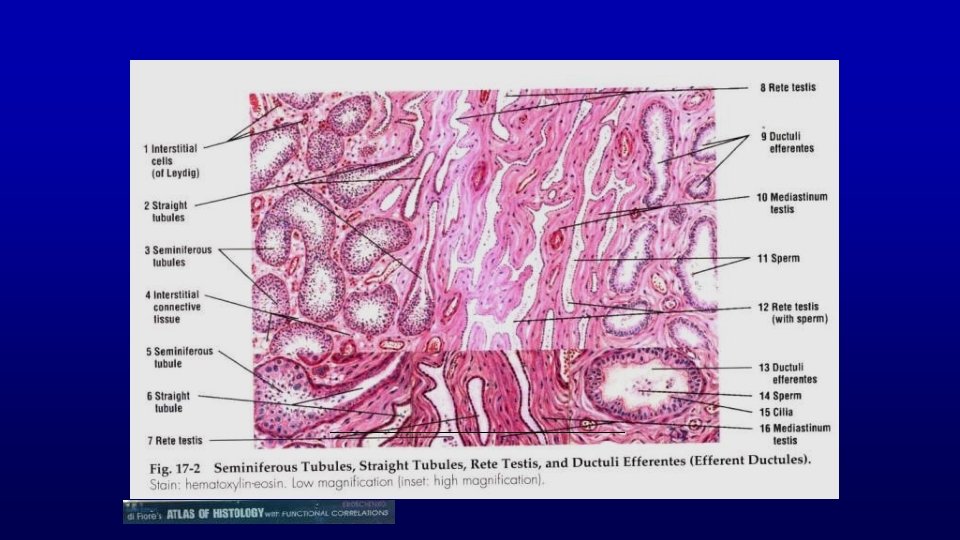
Human testis toluidine blue 19680 Seminiferous epithelium Seminiferous tubules Leydig cells Spermatozoa are produced in the seminiferous epithelium lining the lumen of the testicular tubules. Testosterone is synthesized by Leydig cells located between seminiferous tubules.

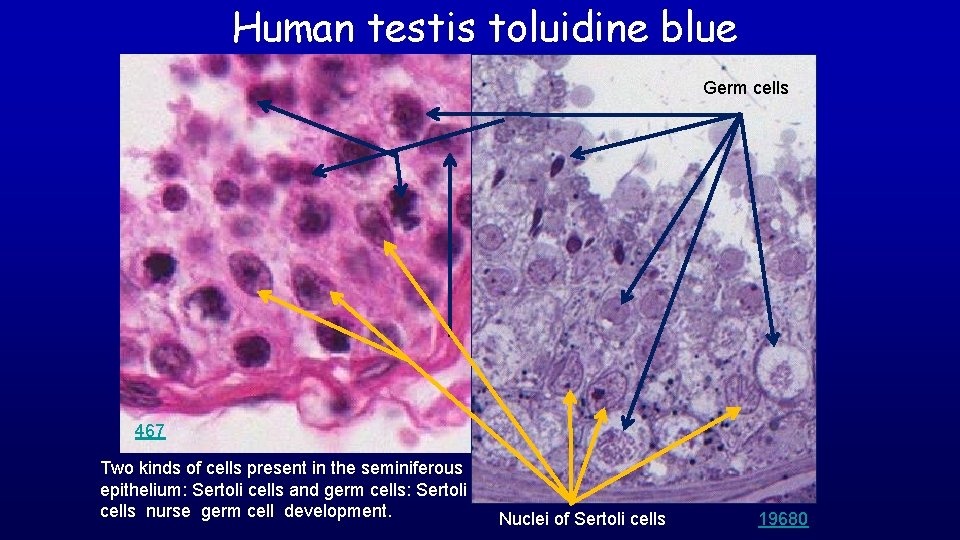
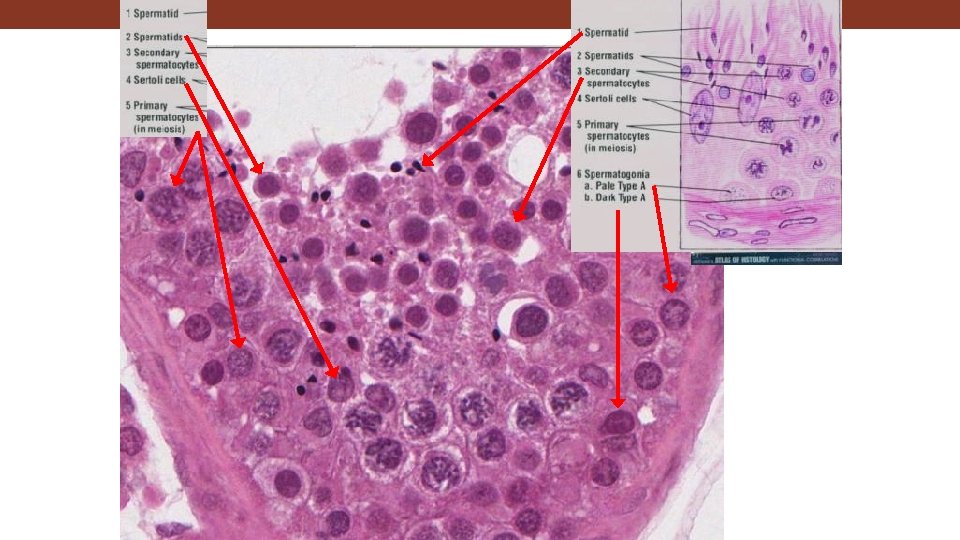
Human testis toluidine blue Germ cells 467 Two kinds of cells present in the seminiferous epithelium: Sertoli cells and germ cells: Sertoli cells nurse germ cell development. Nuclei of Sertoli cells 19680

Horse Seminiferous Tubules

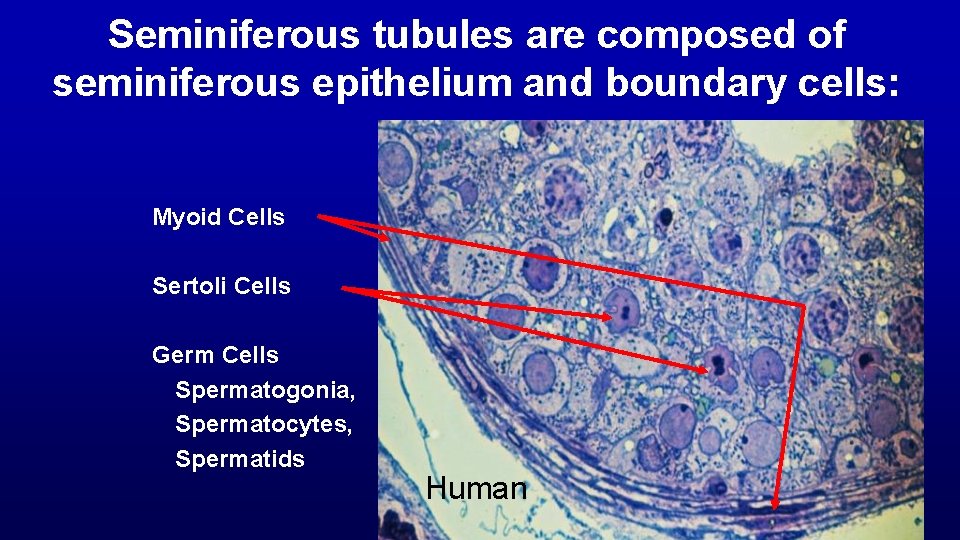
Seminiferous tubules are composed of seminiferous epithelium and boundary cells: Myoid Cells Sertoli Cells Germ Cells Spermatogonia, Spermatocytes, Spermatids Human

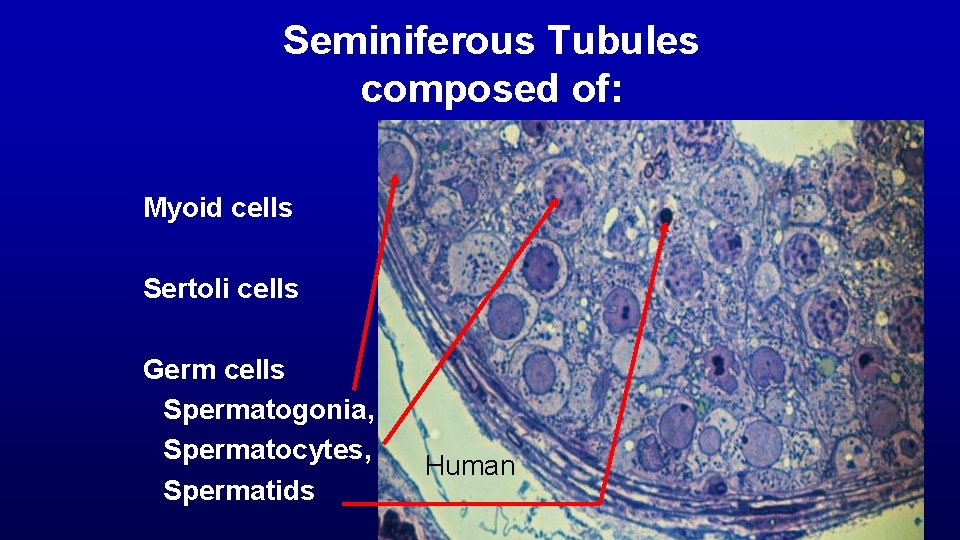
Seminiferous Tubules composed of: Myoid cells Sertoli cells Germ cells Spermatogonia, Spermatocytes, Spermatids Human


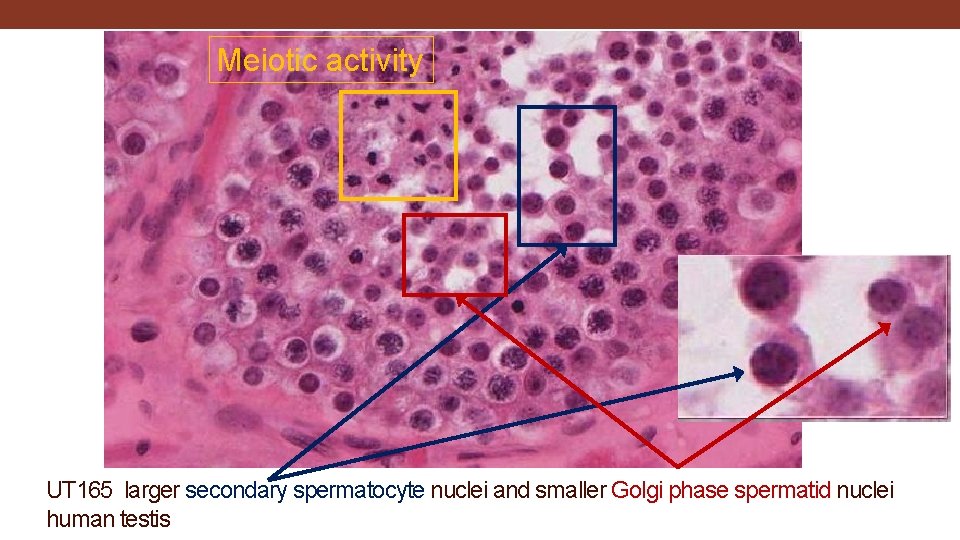
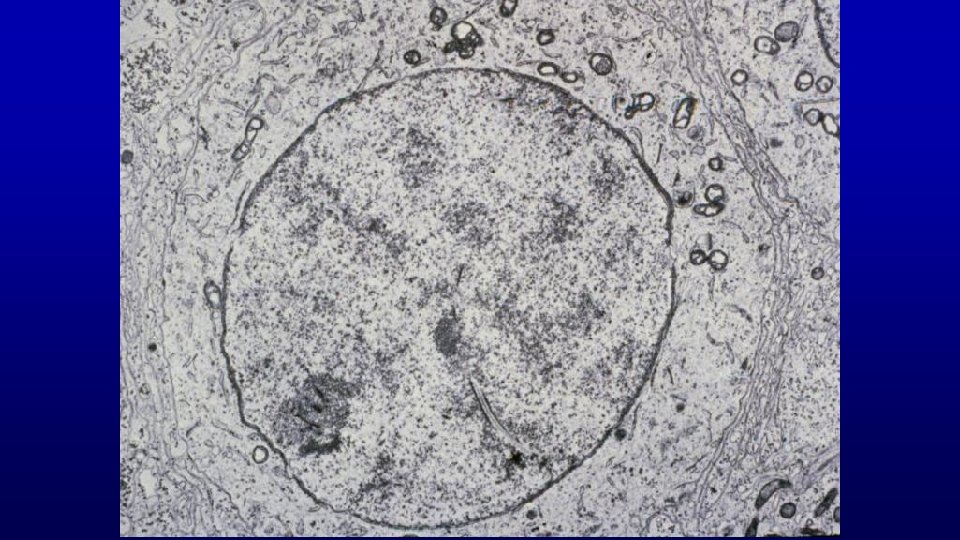
Meiotic activity UT 165 larger secondary spermatocyte nuclei and smaller Golgi phase spermatid nuclei human testis

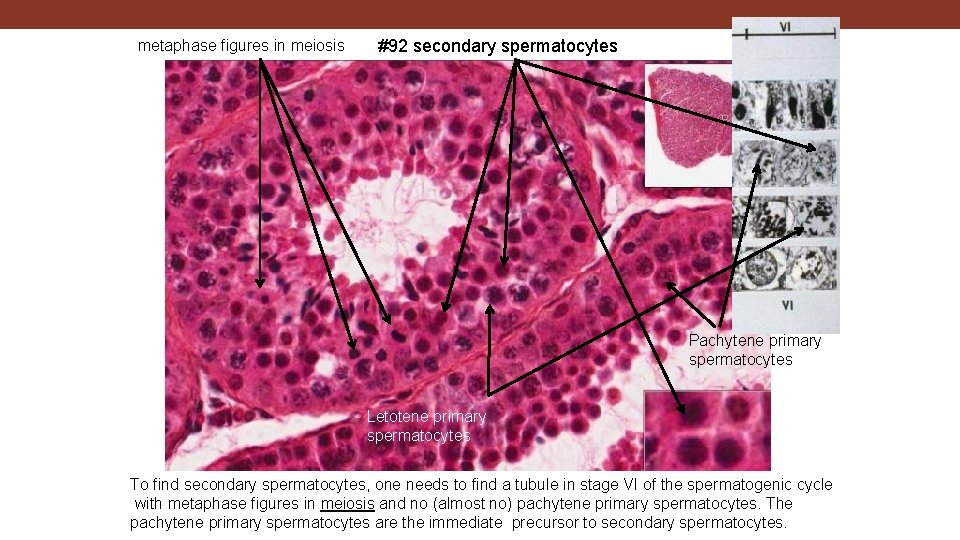
metaphase figures in meiosis #92 secondary spermatocytes Pachytene primary spermatocytes Letotene primary spermatocytes To find secondary spermatocytes, one needs to find a tubule in stage VI of the spermatogenic cycle with metaphase figures in meiosis and no (almost no) pachytene primary spermatocytes. The pachytene primary spermatocytes are the immediate precursor to secondary spermatocytes.

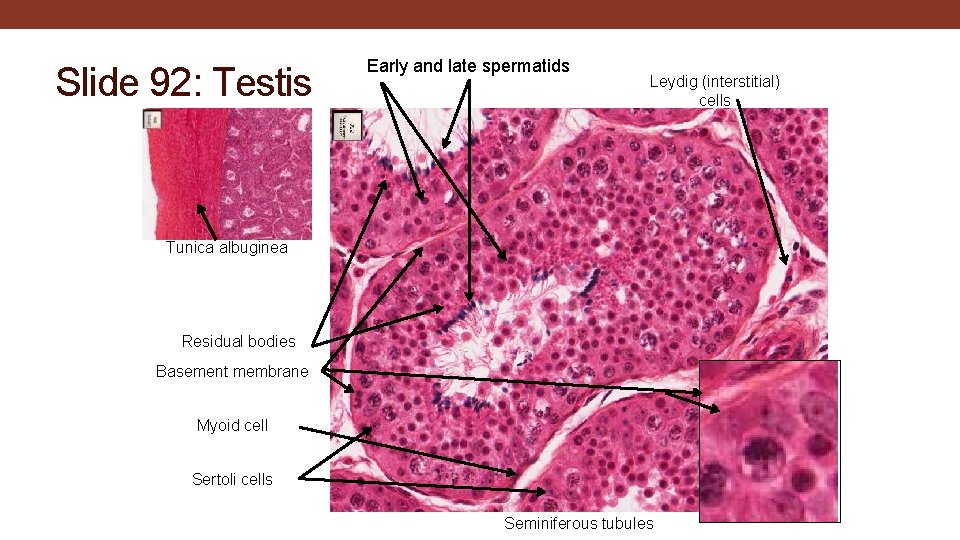
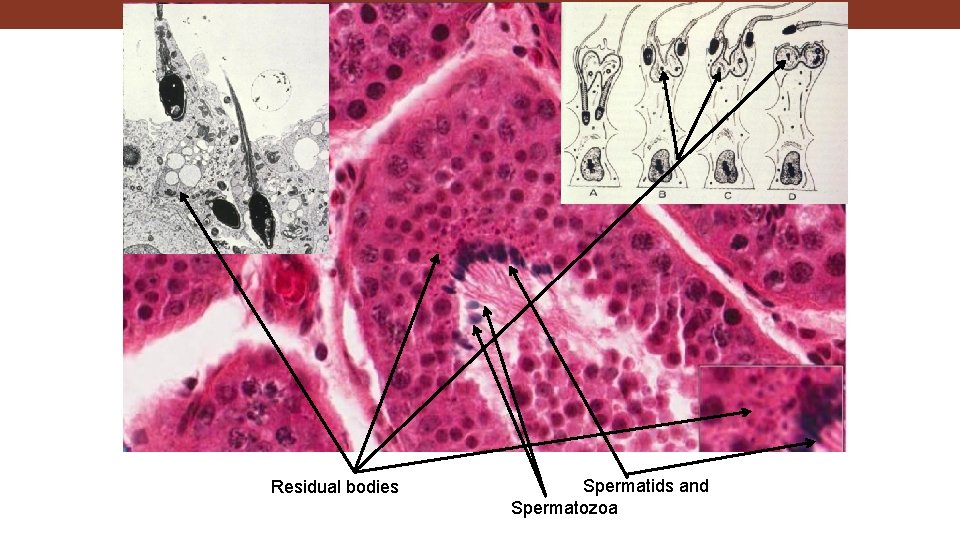
Slide 92: Testis Early and late spermatids Leydig (interstitial) cells Tunica albuginea Residual bodies Basement membrane Myoid cell Sertoli cells Seminiferous tubules

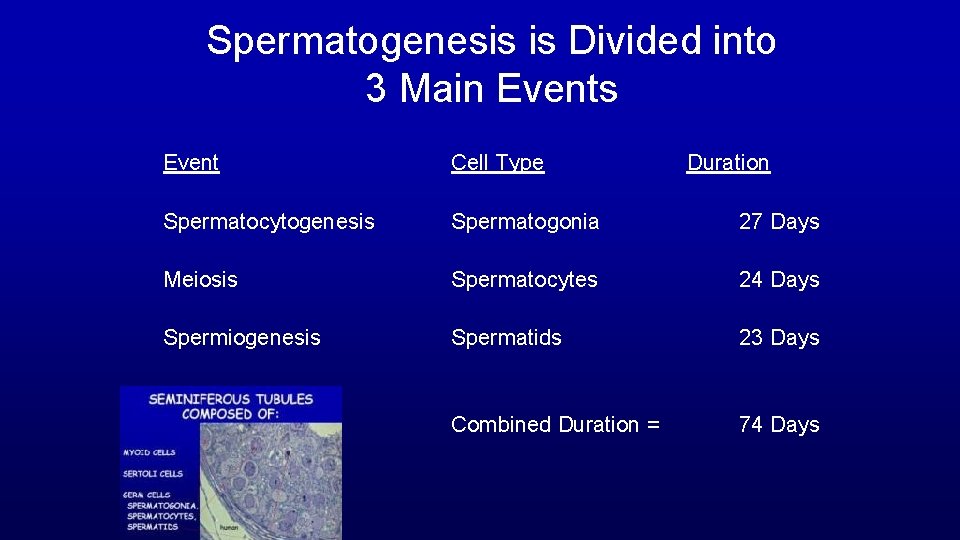
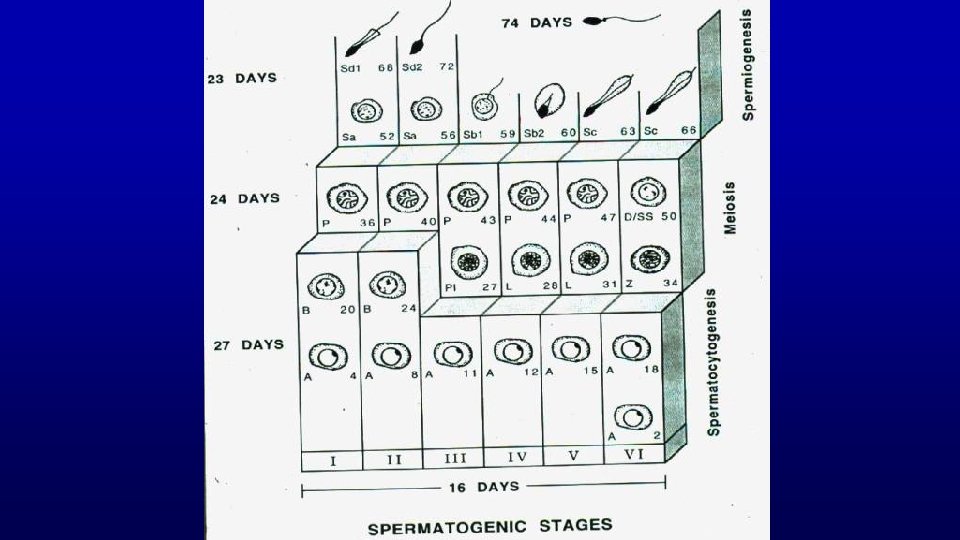
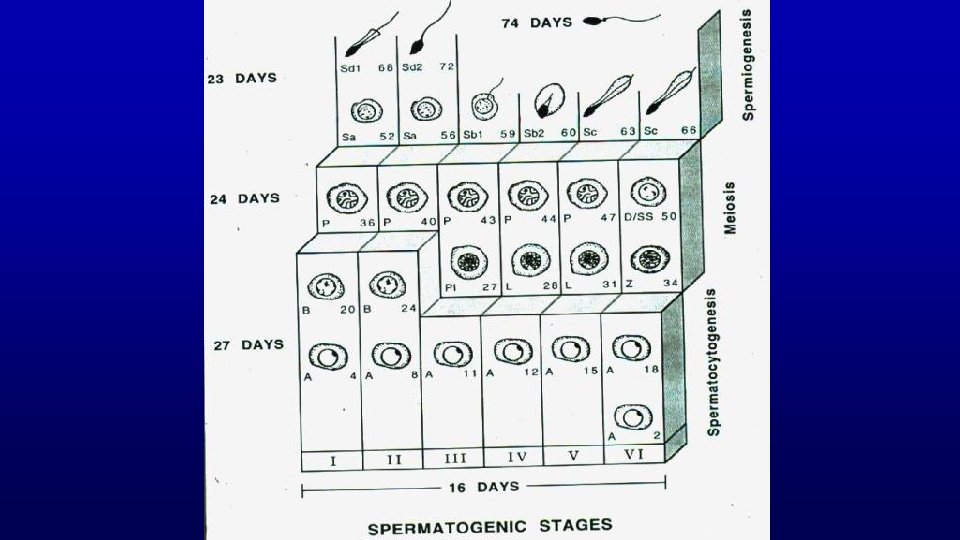
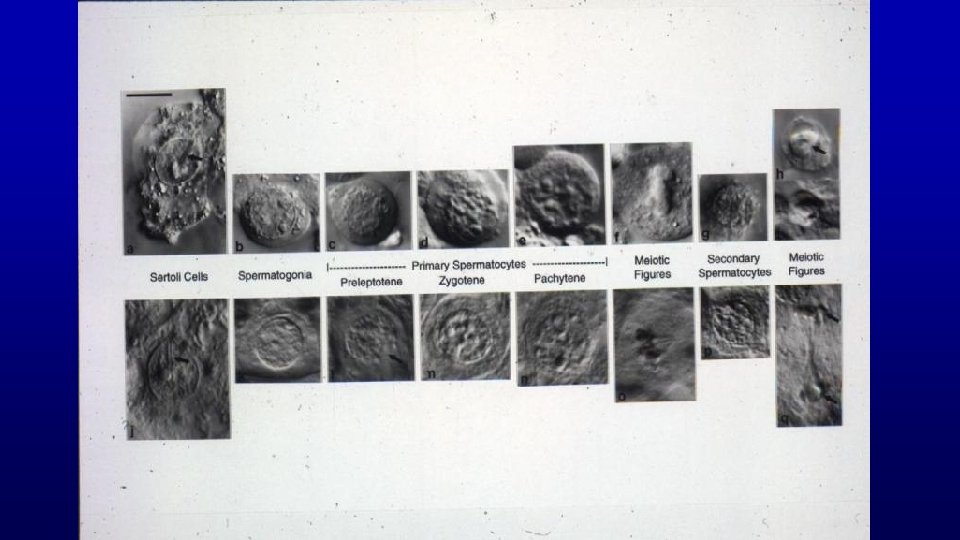
Spermatogenesis is Divided into 3 Main Events Event Cell Type Duration Spermatocytogenesis Spermatogonia 27 Days Meiosis Spermatocytes 24 Days Spermiogenesis Spermatids 23 Days Combined Duration = 74 Days

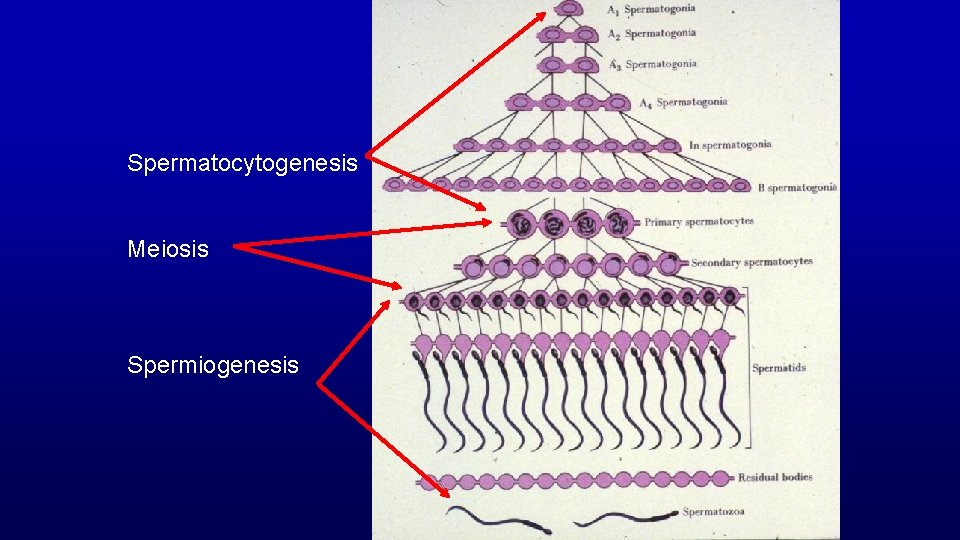
Spermatocytogenesis Meiosis Spermiogenesis

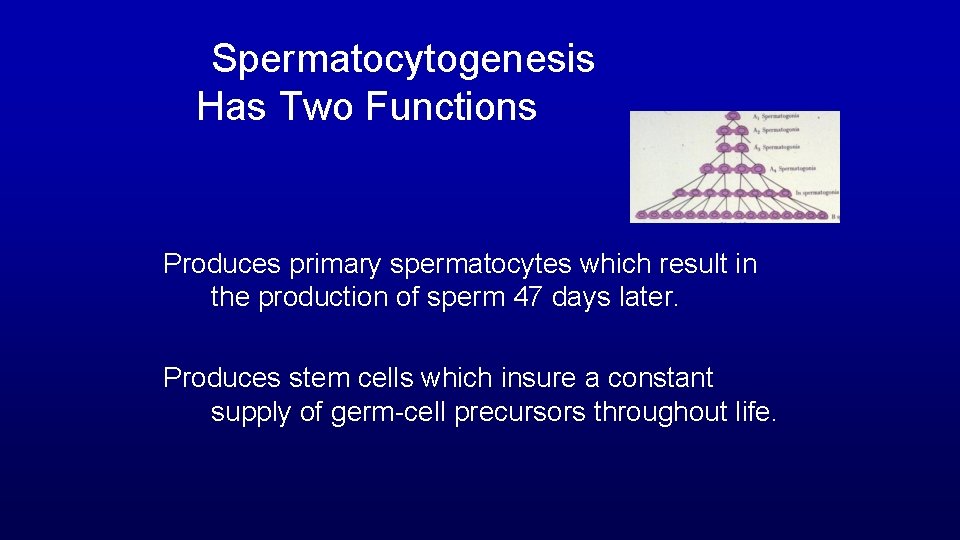
Spermatocytogenesis Has Two Functions Produces primary spermatocytes which result in the production of sperm 47 days later. Produces stem cells which insure a constant supply of germ-cell precursors throughout life.

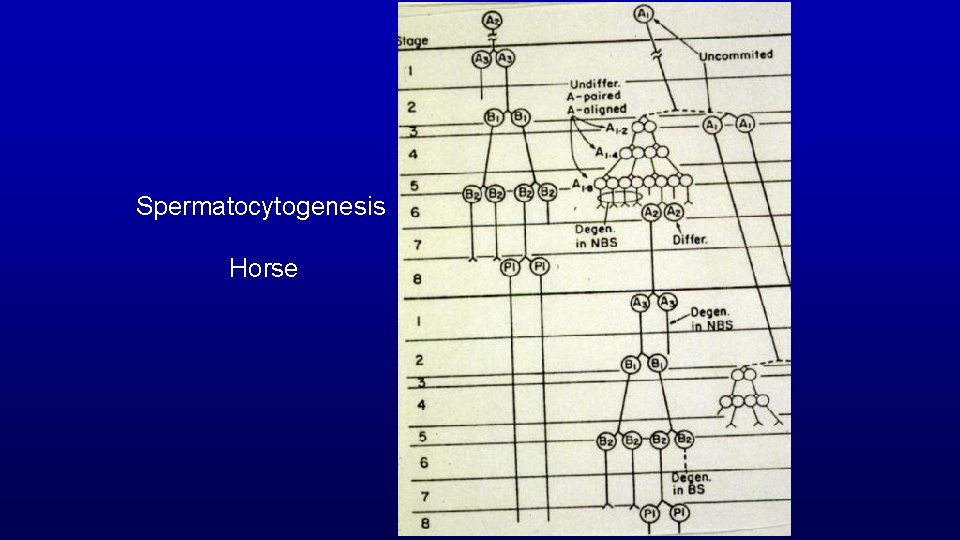
Spermatocytogenesis Horse

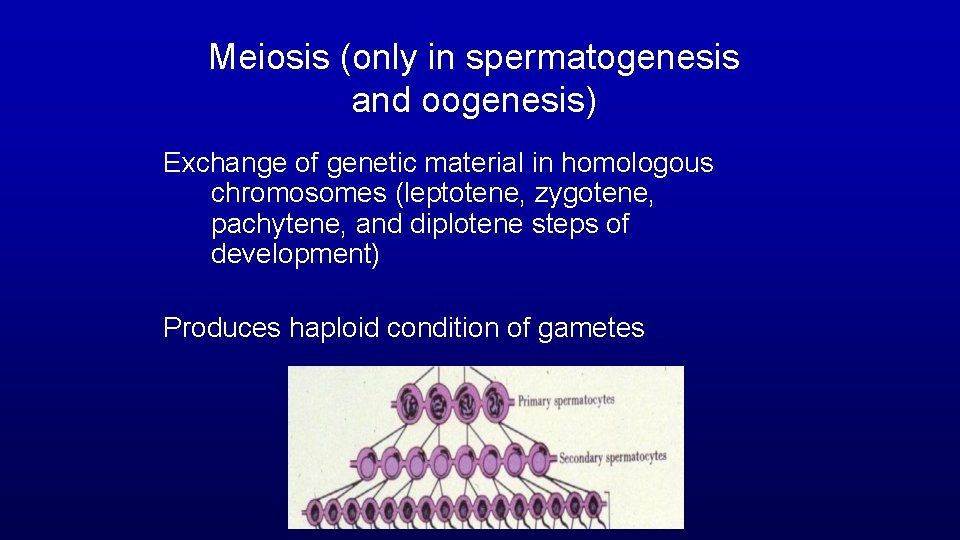
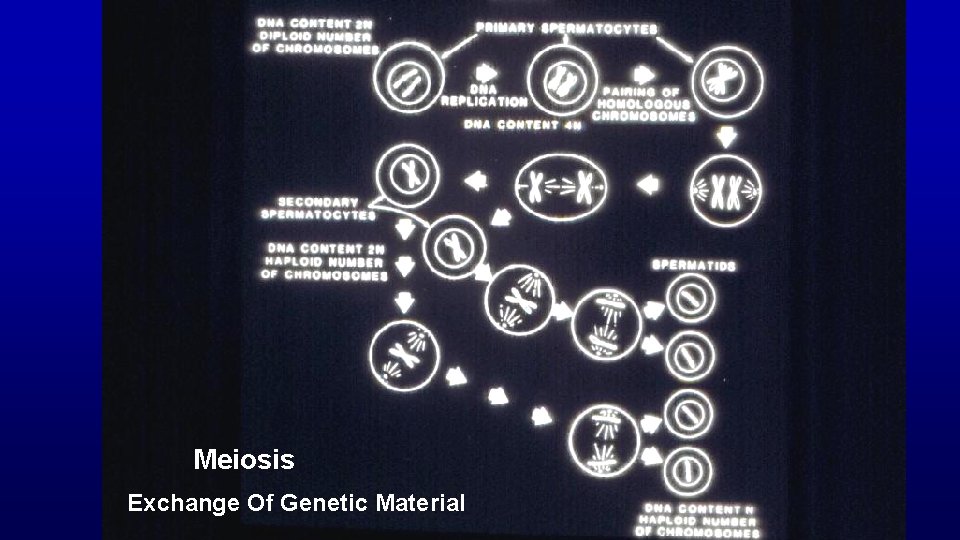
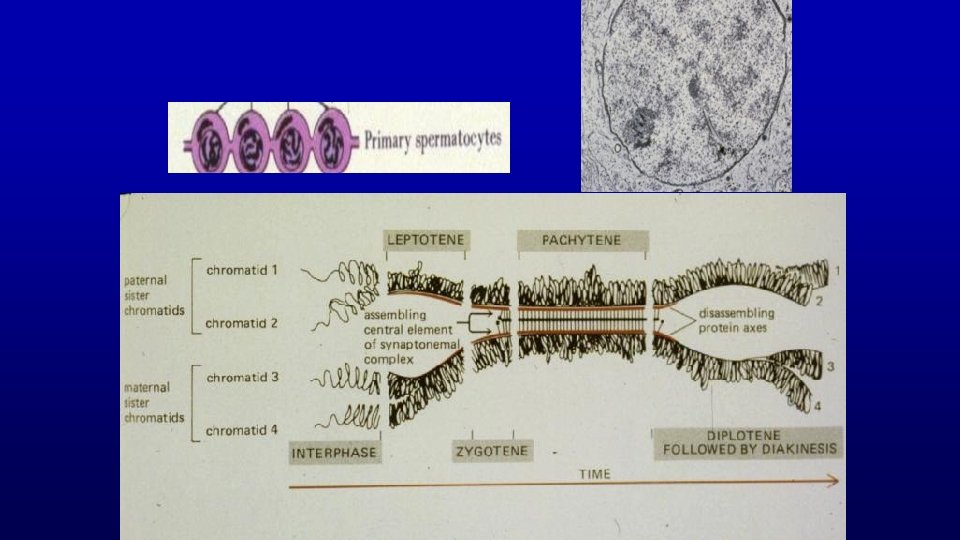
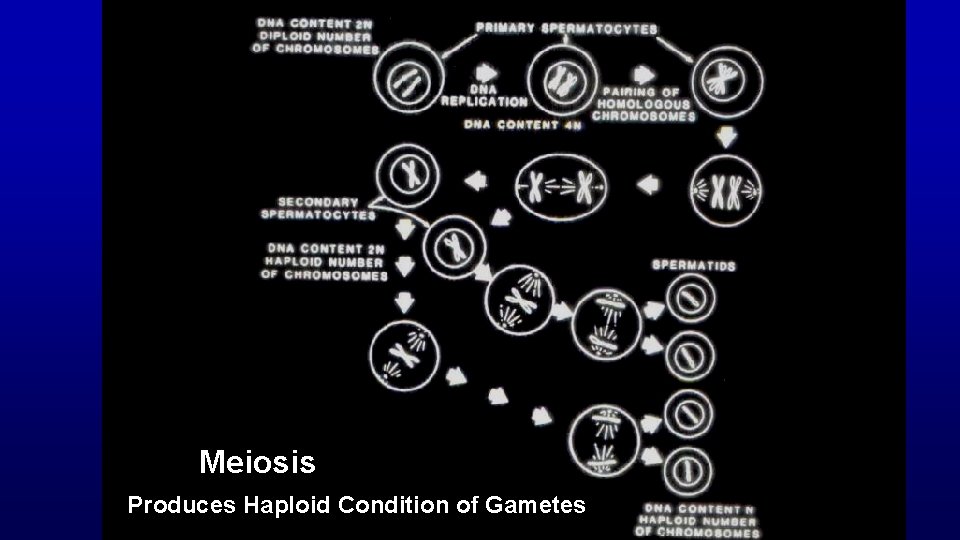
Meiosis (only in spermatogenesis and oogenesis) Exchange of genetic material in homologous chromosomes (leptotene, zygotene, pachytene, and diplotene steps of development) Produces haploid condition of gametes

Meiosis Exchange Of Genetic Material




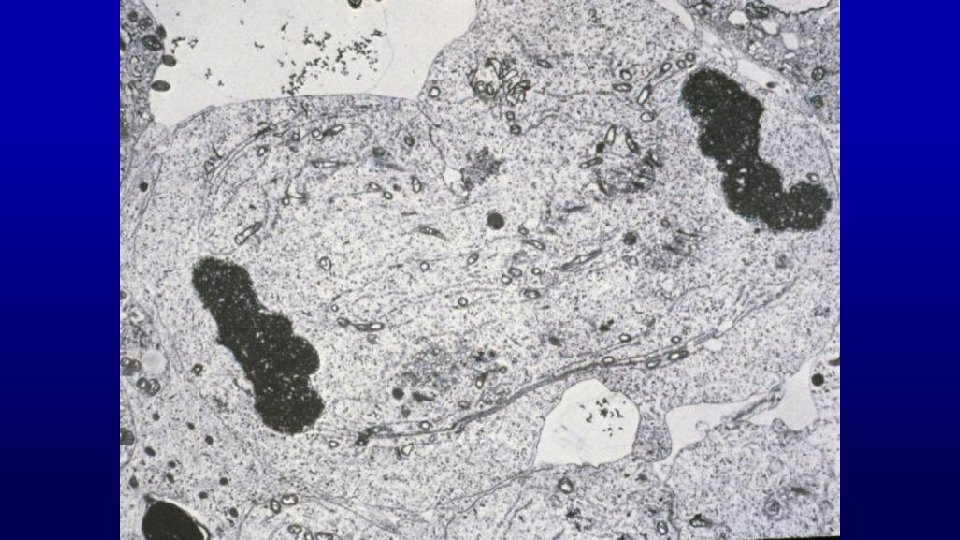
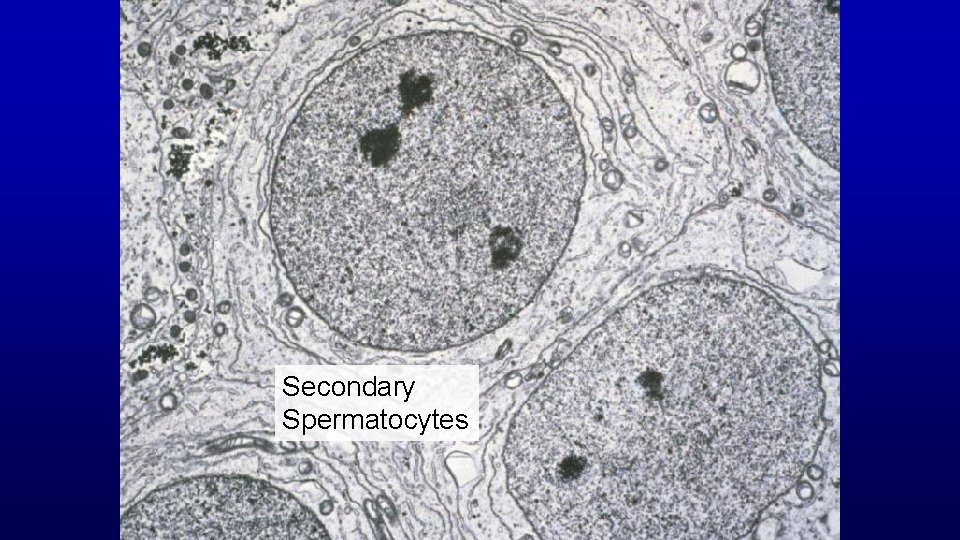
Secondary Spermatocytes

Meiosis Produces Haploid Condition of Gametes

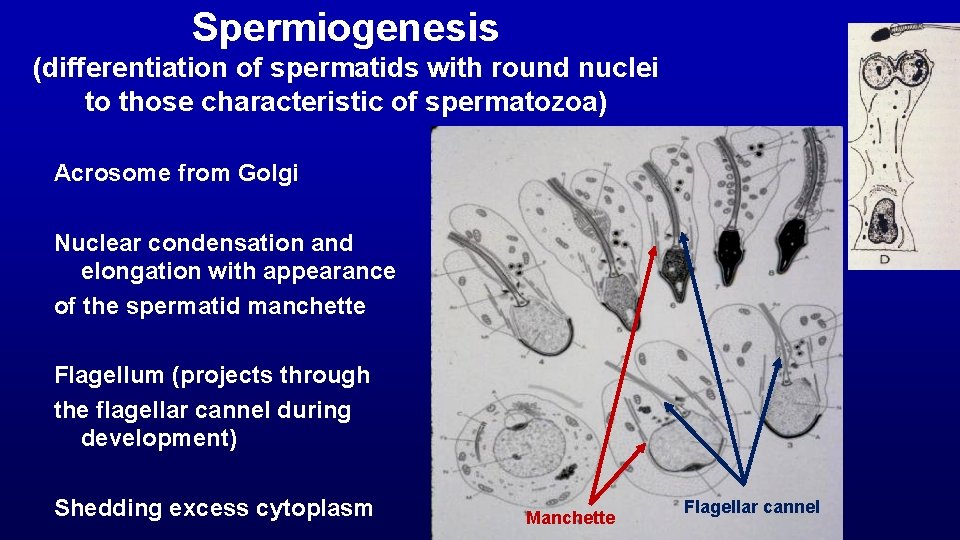
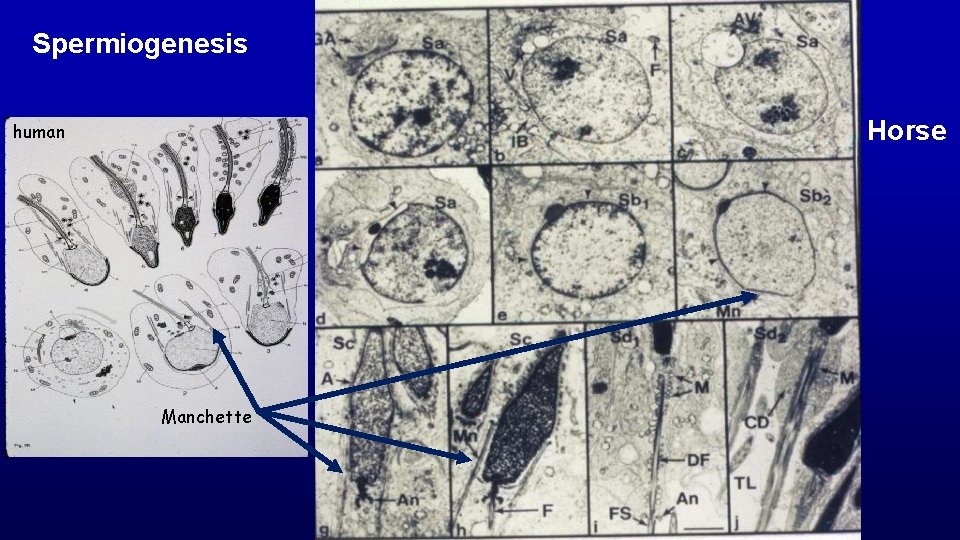
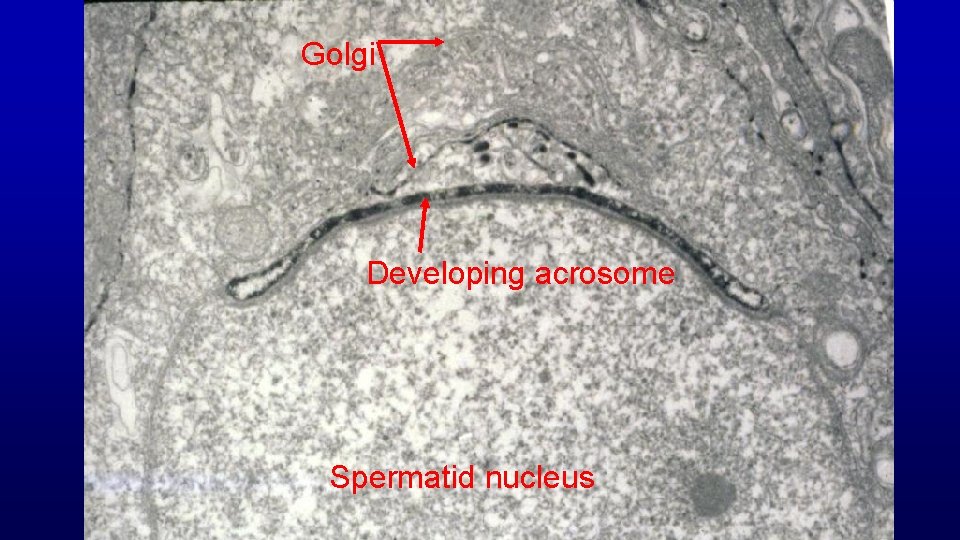
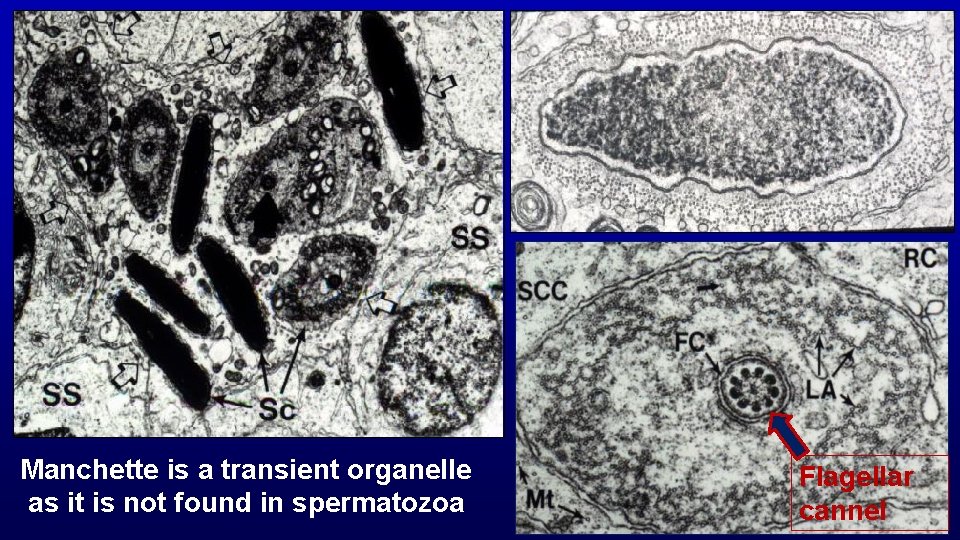
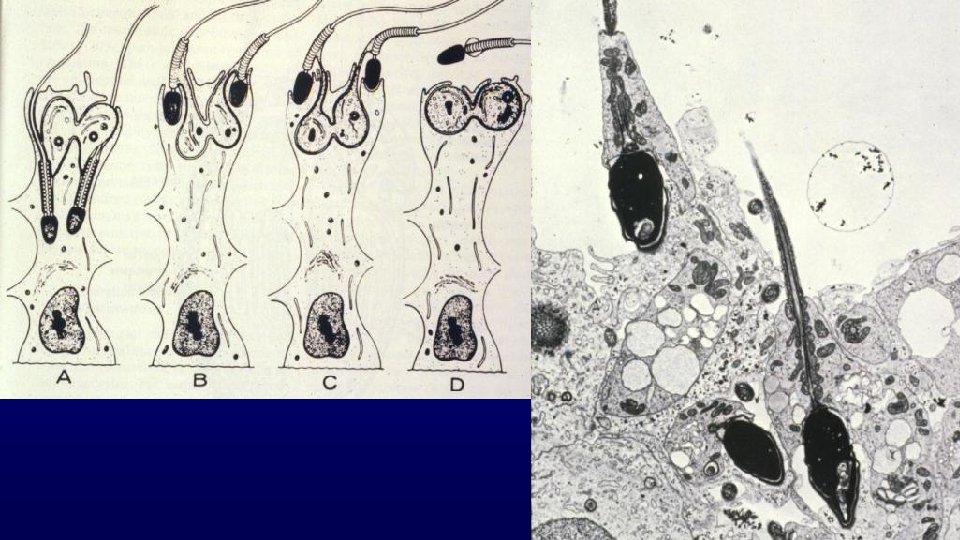
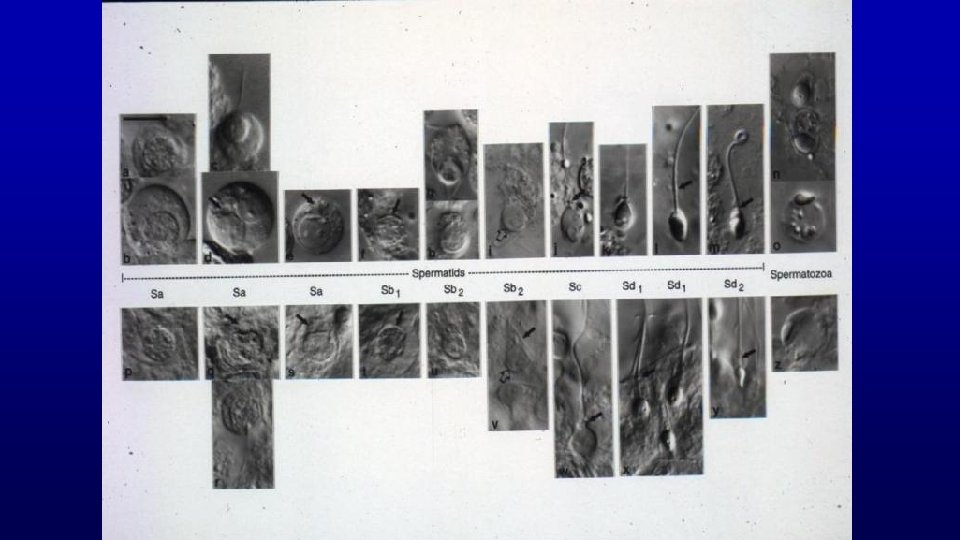
Spermiogenesis (differentiation of spermatids with round nuclei to those characteristic of spermatozoa) Acrosome from Golgi Nuclear condensation and elongation with appearance of the spermatid manchette Flagellum (projects through the flagellar cannel during development) Shedding excess cytoplasm Manchette Flagellar cannel

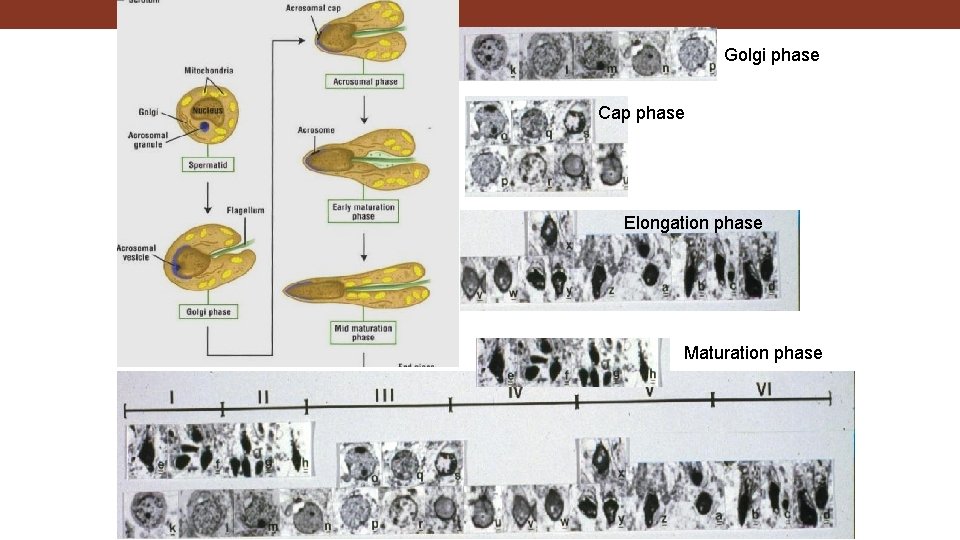
Golgi phase Cap phase Elongation phase Maturation phase

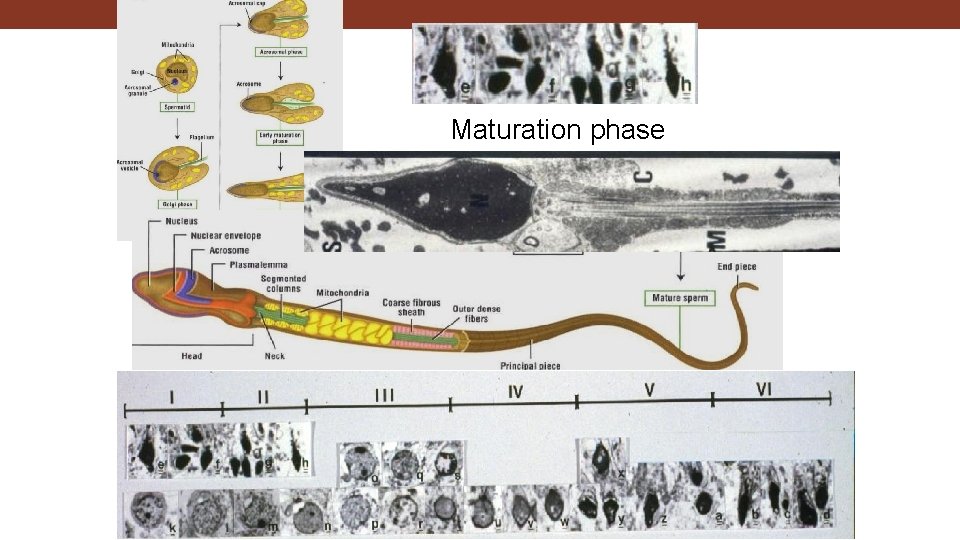
Maturation phase

Spermiogenesis Horse human Manchette

Golgi Developing acrosome Spermatid nucleus

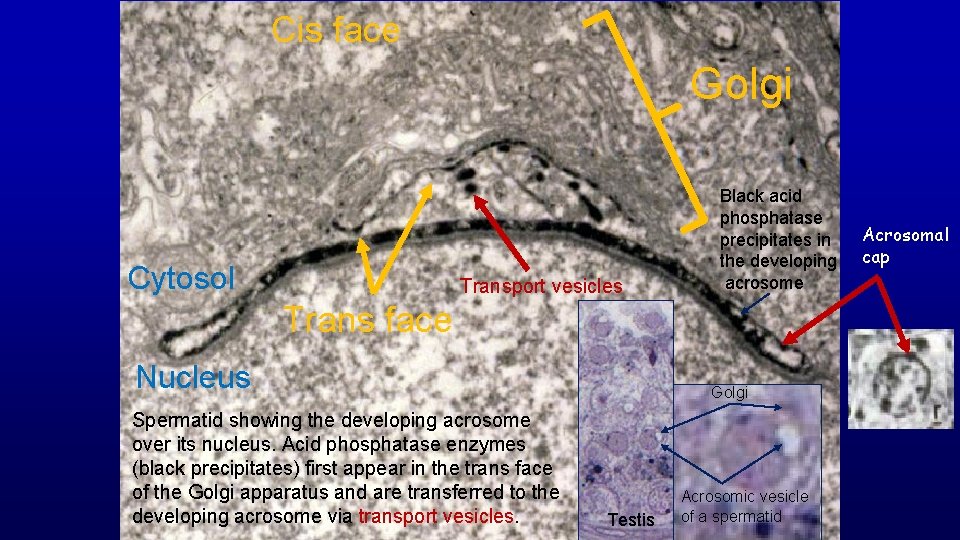
Cis face Golgi Cytosol Transport vesicles Black acid phosphatase precipitates in the developing acrosome Trans face Nucleus Spermatid showing the developing acrosome over its nucleus. Acid phosphatase enzymes (black precipitates) first appear in the trans face of the Golgi apparatus and are transferred to the developing acrosome via transport vesicles. Golgi Testis Acrosomic vesicle of a spermatid Acrosomal cap

Manchette is a transient organelle as it is not found in spermatozoa Flagellar cannel

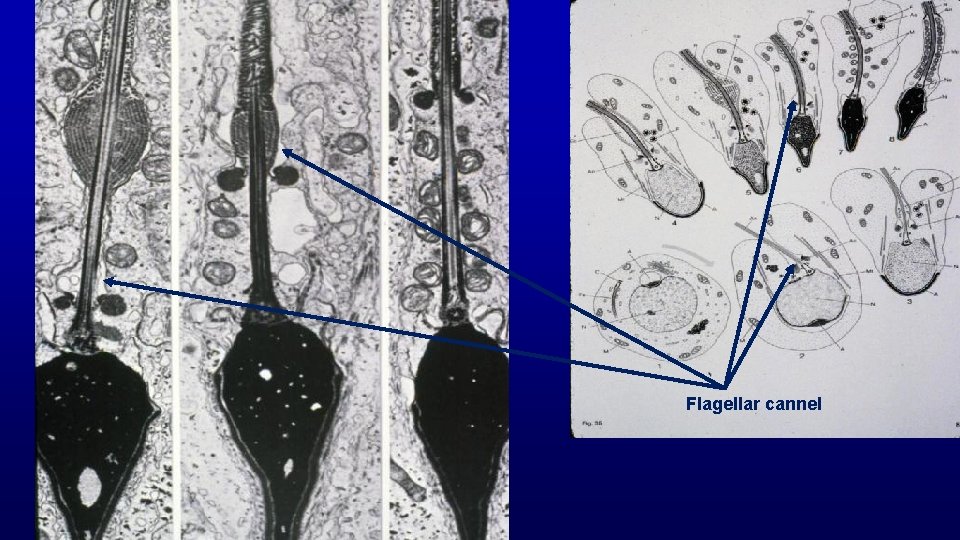
Flagellar cannel

Annulus Flagellar cannel When the annulus migrates to end of middle piece, it removes the cell membrane of the flagellar cannel from the surface of the developing flagellum in the middle piece region and allows mitochondria access to that portion of developing tail. Mitochondria are found only in the middle piece on spermatozoa as that is the only region with access.



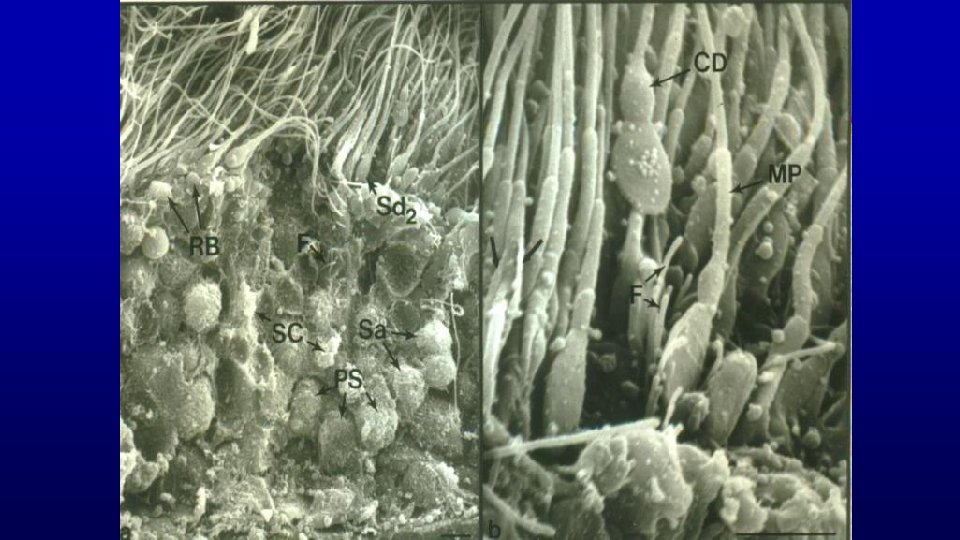
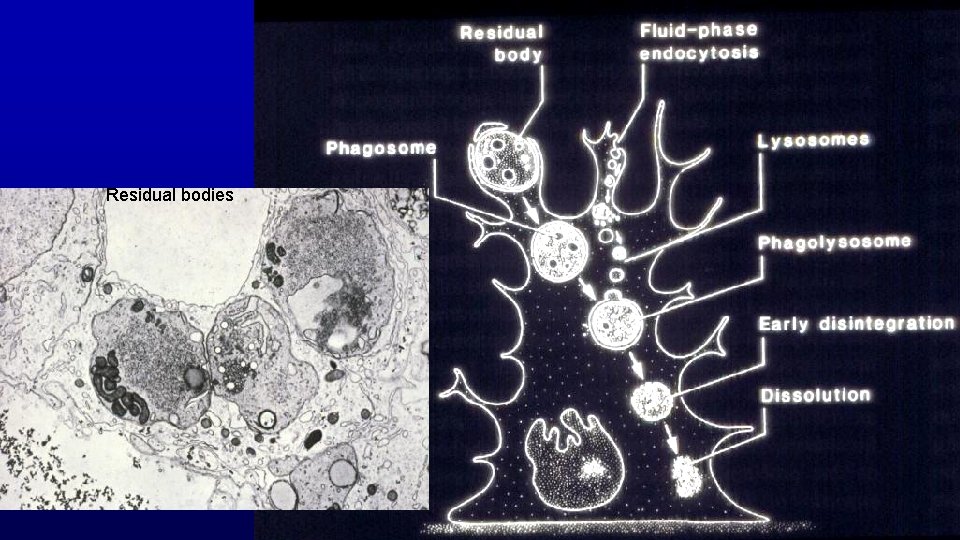
Residual bodies Spermatids and Spermatozoa

Residual bodies

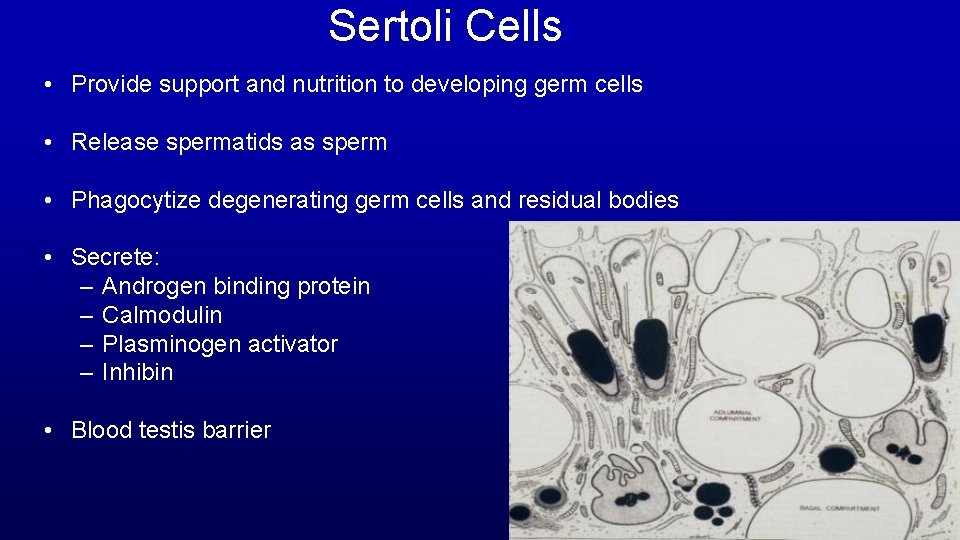
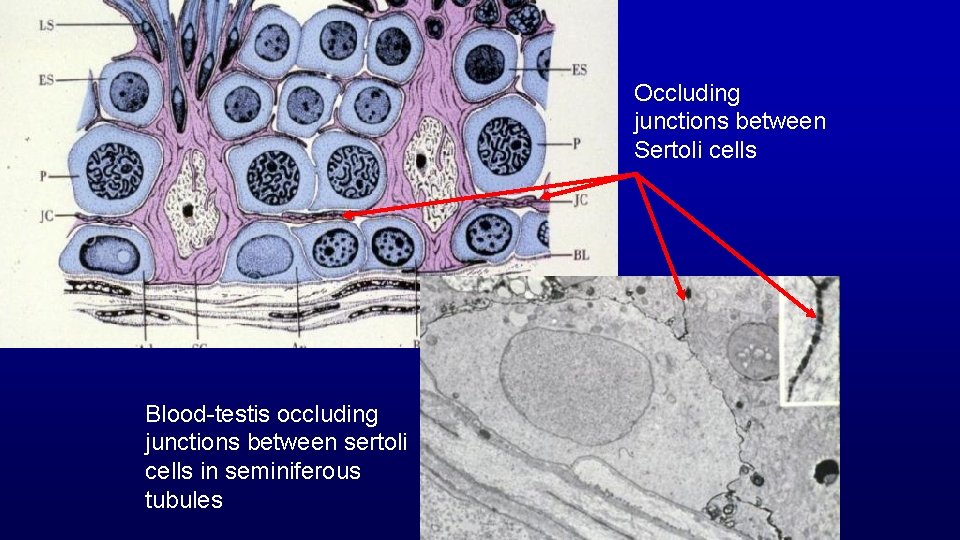
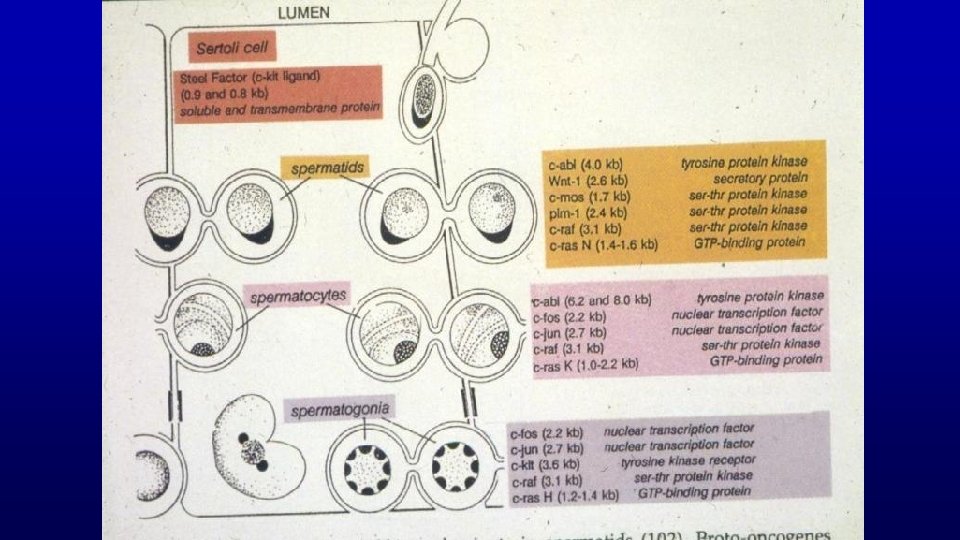
Sertoli Cells • Provide support and nutrition to developing germ cells • Release spermatids as sperm • Phagocytize degenerating germ cells and residual bodies • Secrete: – Androgen binding protein – Calmodulin – Plasminogen activator – Inhibin • Blood testis barrier

Human – infertile man



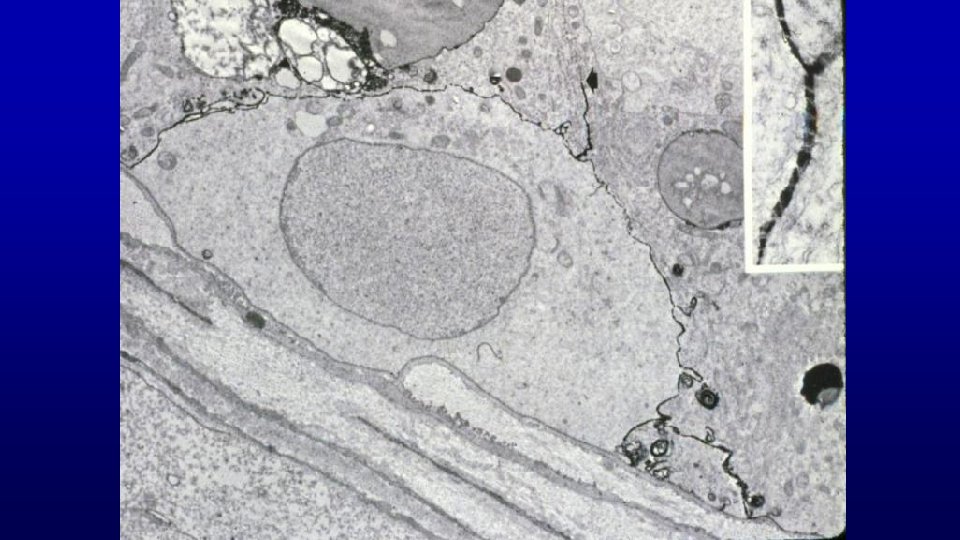
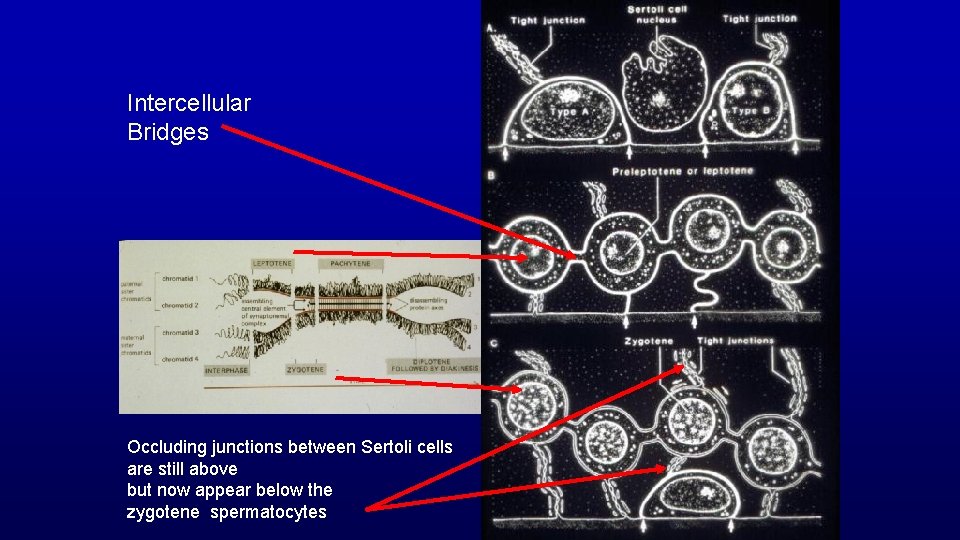
Occluding junctions between Sertoli cells Blood-testis occluding junctions between sertoli cells in seminiferous tubules

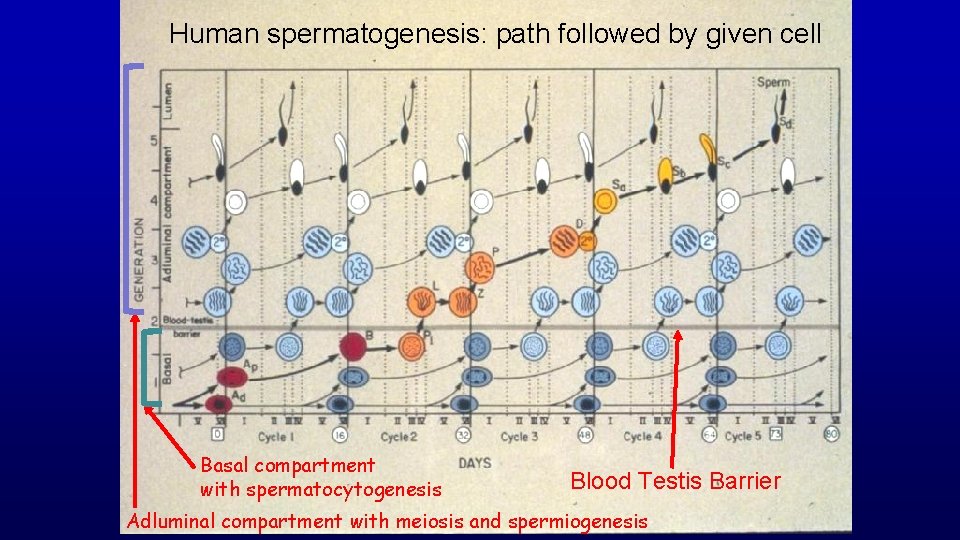
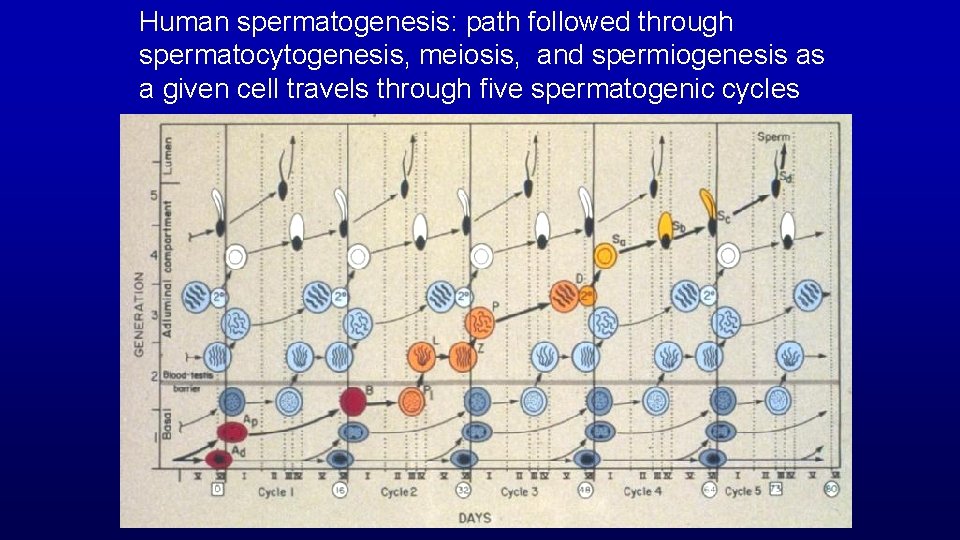
Human spermatogenesis: path followed by given cell Basal compartment with spermatocytogenesis Blood Testis Barrier Adluminal compartment with meiosis and spermiogenesis

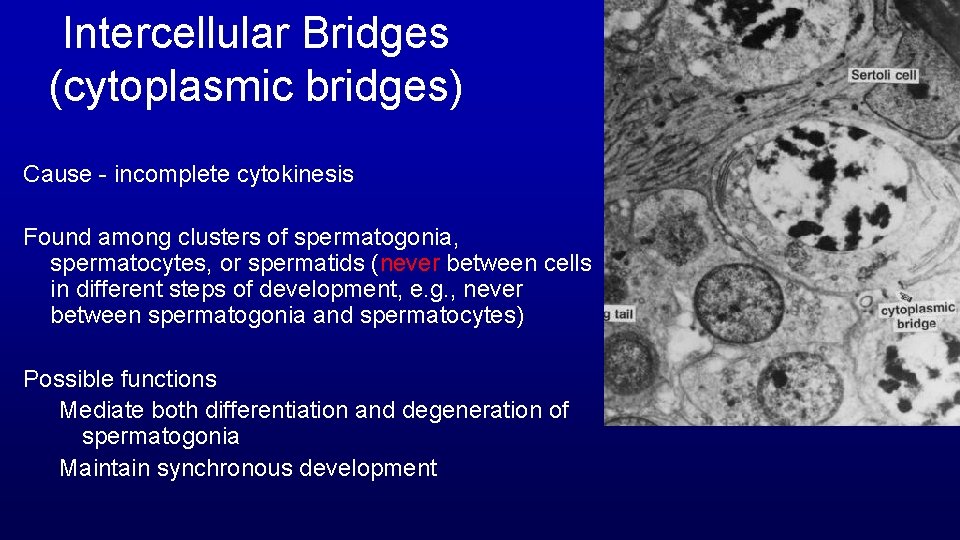
Intercellular Bridges (cytoplasmic bridges) Cause - incomplete cytokinesis Found among clusters of spermatogonia, spermatocytes, or spermatids (never between cells in different steps of development, e. g. , never between spermatogonia and spermatocytes) Possible functions Mediate both differentiation and degeneration of spermatogonia Maintain synchronous development

Intercellular Bridges Occluding junctions between Sertoli cells are still above but now appear below the zygotene spermatocytes

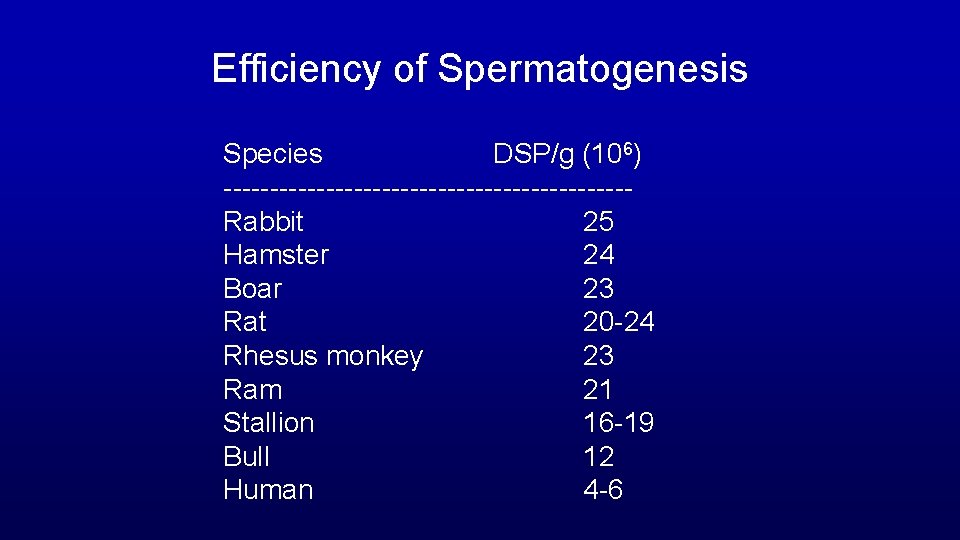
Efficiency of Spermatogenesis Species DSP/g (106) ----------------------Rabbit 25 Hamster 24 Boar 23 Rat 20 -24 Rhesus monkey 23 Ram 21 Stallion 16 -19 Bull 12 Human 4 -6

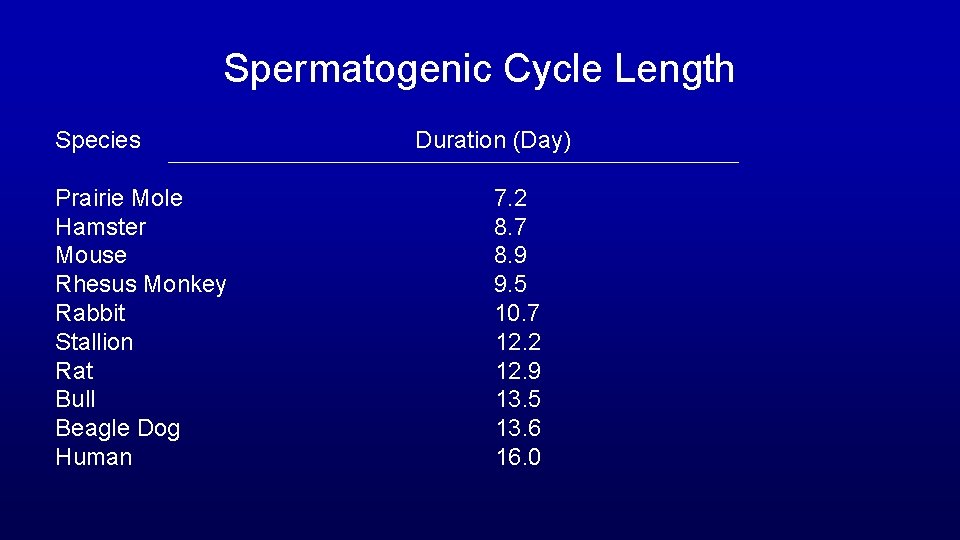
Spermatogenic Cycle Length Species Duration (Day) Prairie Mole Hamster Mouse Rhesus Monkey Rabbit Stallion Rat Bull Beagle Dog Human 7. 2 8. 7 8. 9 9. 5 10. 7 12. 2 12. 9 13. 5 13. 6 16. 0

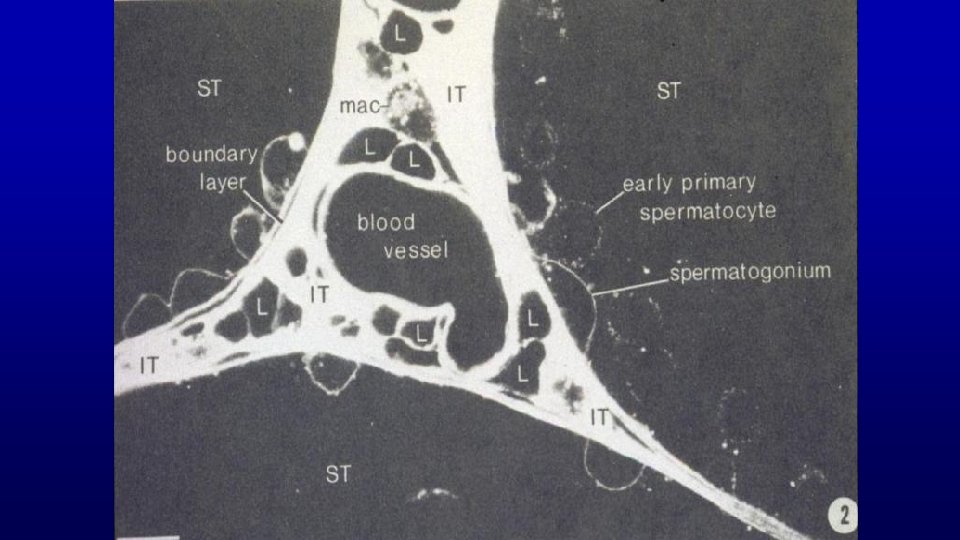
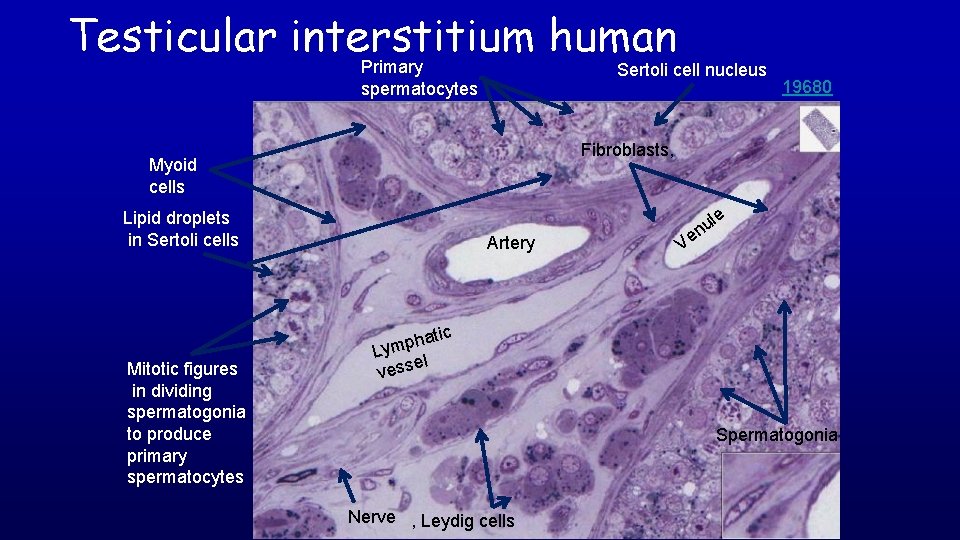
Testicular interstitium human Primary spermatocytes Sertoli cell nucleus 19680 Fibroblasts, Myoid cells Lipid droplets in Sertoli cells Artery le u n Ve c Mitotic figures in dividing spermatogonia to produce primary spermatocytes hati p m y L el vess Spermatogonia Nerve , Leydig cells

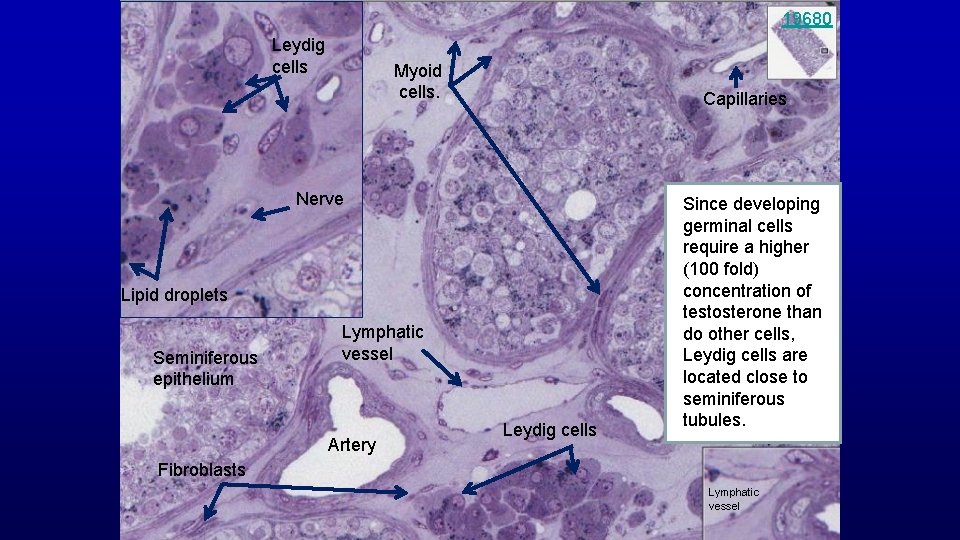
19680 Leydig cells Myoid cells. Capillaries Nerve Lipid droplets Seminiferous epithelium Lymphatic vessel Artery Leydig cells Since developing germinal cells require a higher (100 fold) concentration of testosterone than do other cells, Leydig cells are located close to seminiferous tubules. Fibroblasts Lymphatic vessel

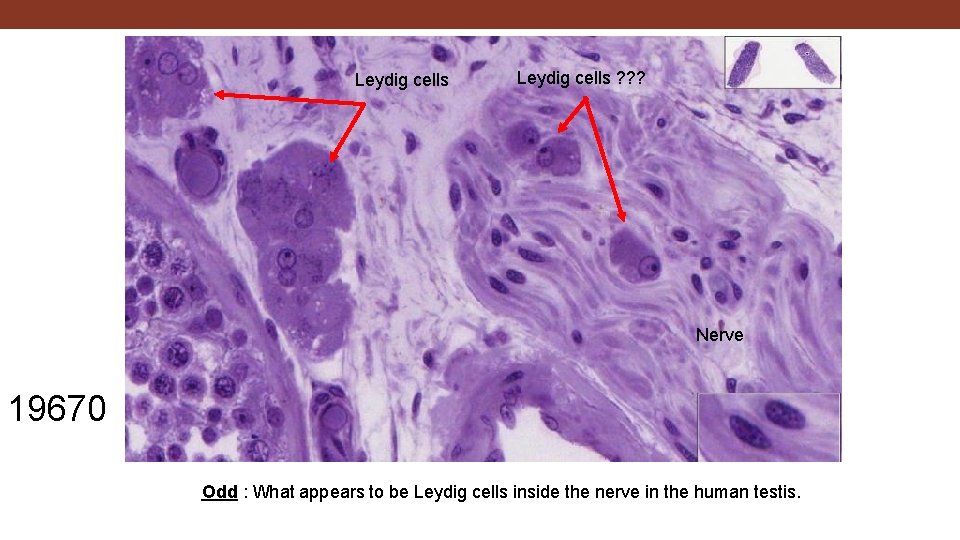
Leydig cells ? ? ? Nerve 19670 Odd : What appears to be Leydig cells inside the nerve in the human testis.


Horse

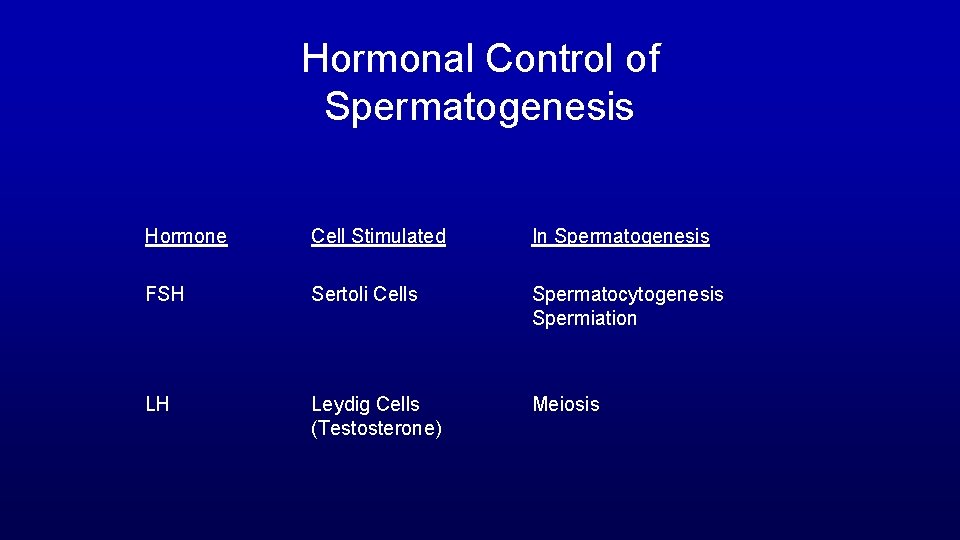
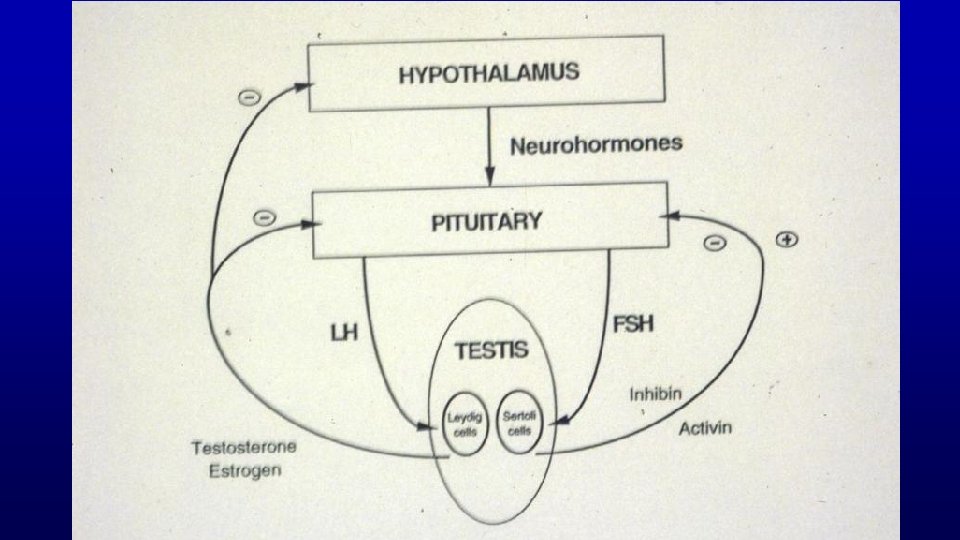
Hormonal Control of Spermatogenesis Hormone Cell Stimulated In Spermatogenesis FSH Sertoli Cells Spermatocytogenesis Spermiation LH Leydig Cells (Testosterone) Meiosis


Deleterious Influences on Spermatogenesis • Heat • Irradiation • Chemicals • Aging


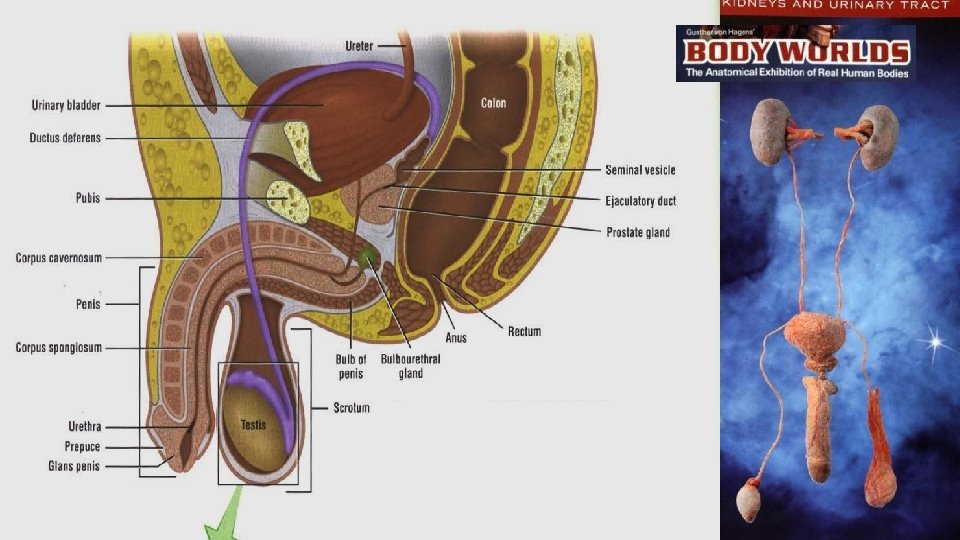
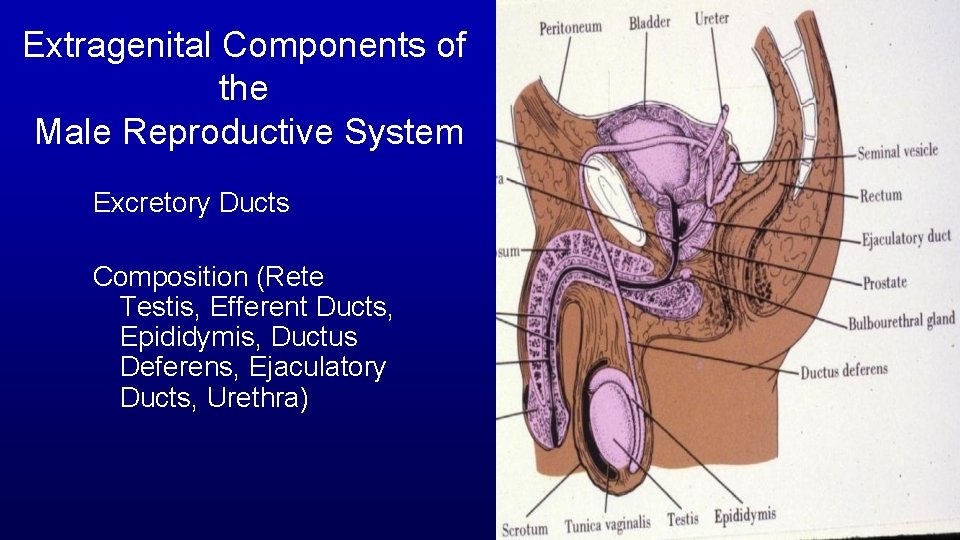
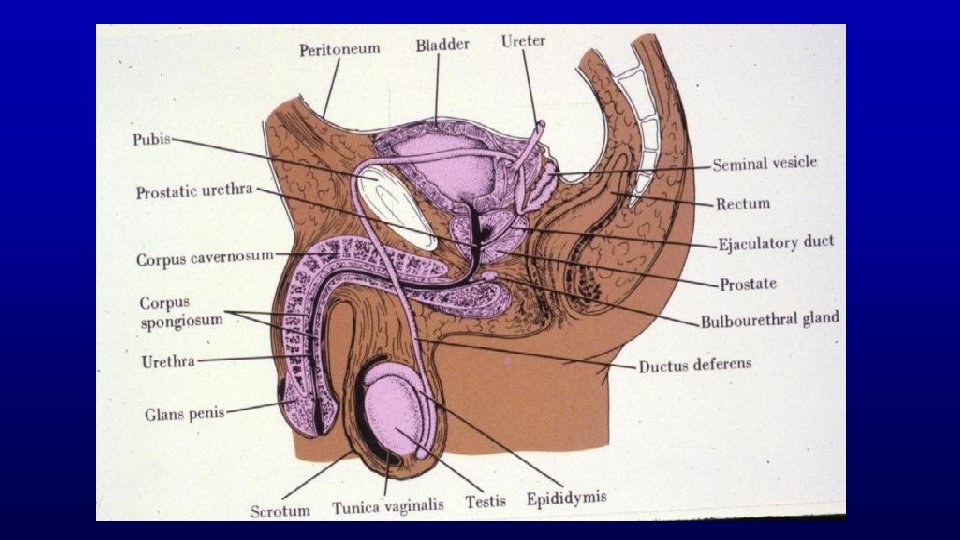
Extragenital Components of the Male Reproductive System Excretory Ducts Composition (Rete Testis, Efferent Ducts, Epididymis, Ductus Deferens, Ejaculatory Ducts, Urethra)



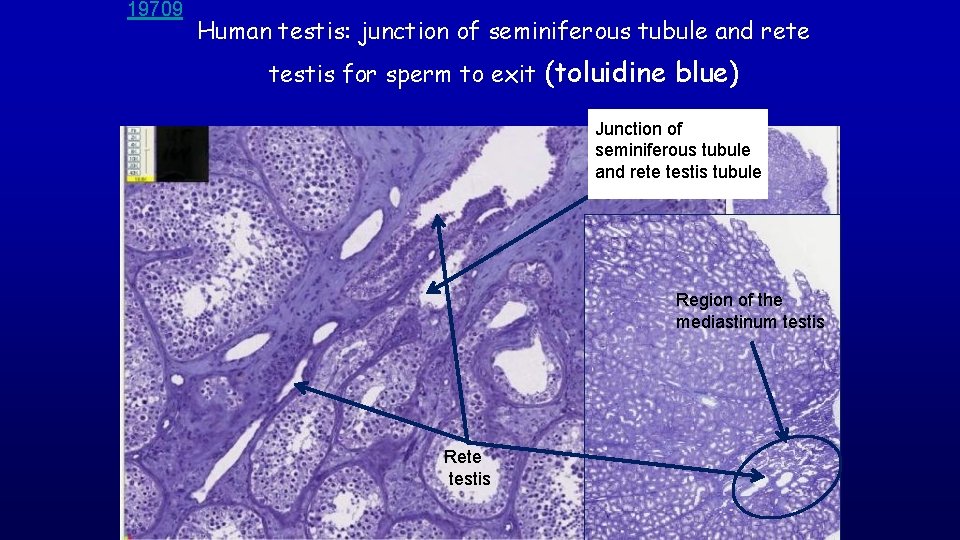
19709 Human testis: junction of seminiferous tubule and rete testis for sperm to exit (toluidine blue) Junction of seminiferous tubule and rete testis tubule Region of the mediastinum testis Rete testis

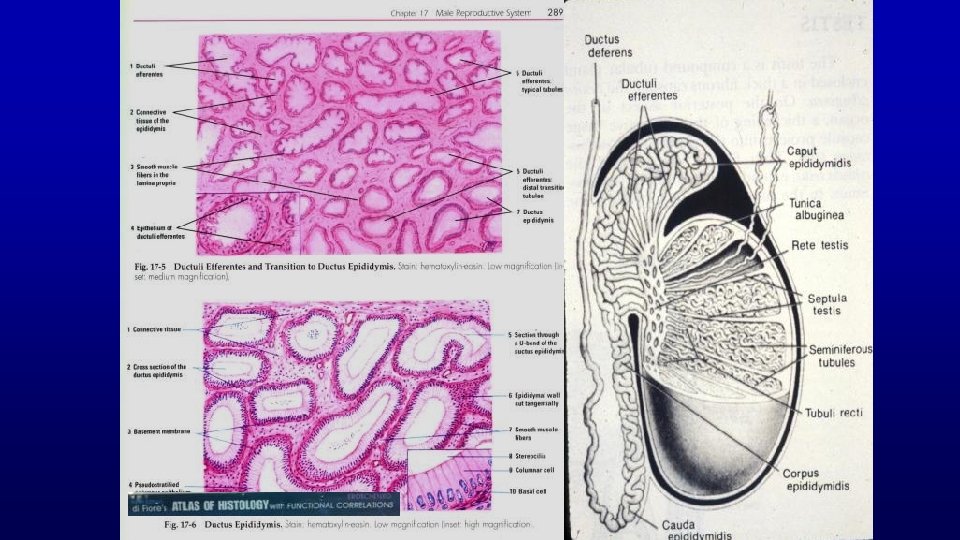
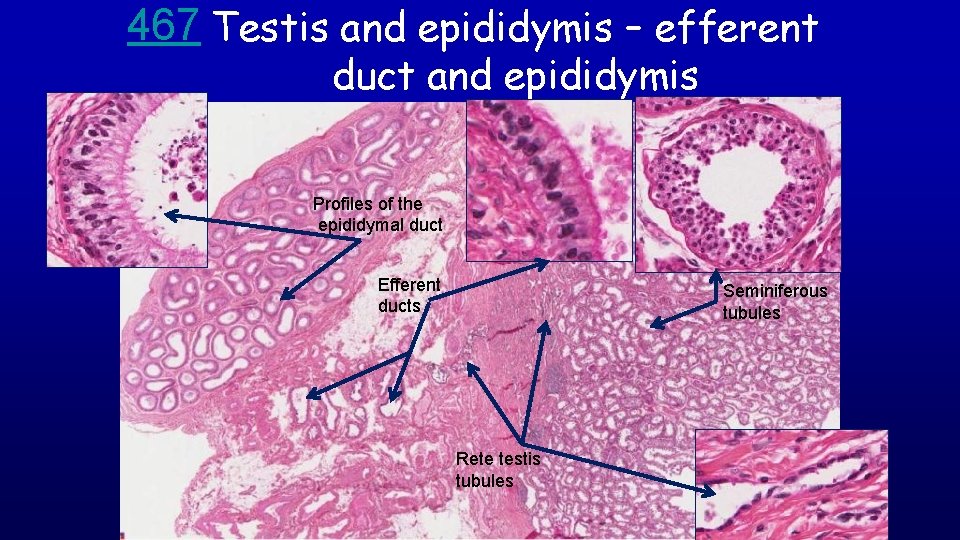
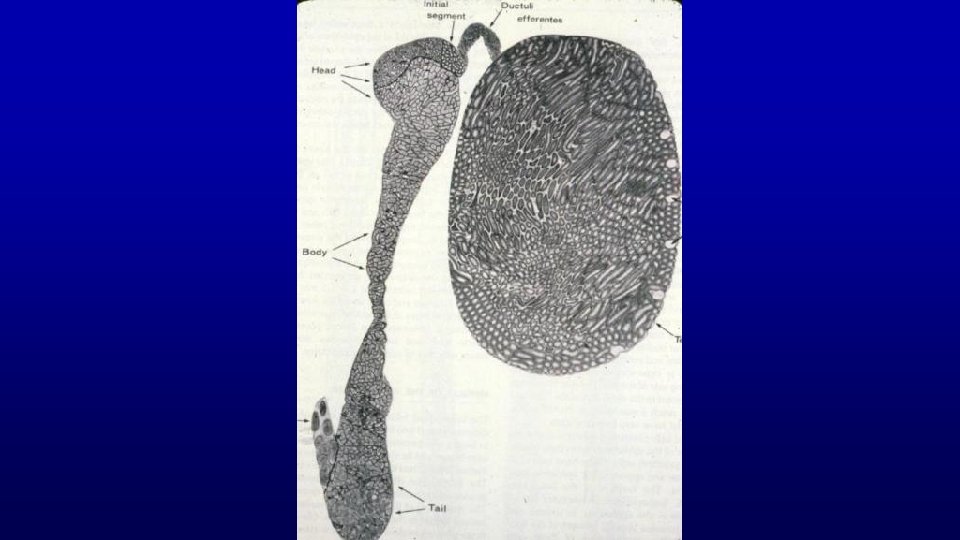
467 Testis and epididymis – efferent duct and epididymis Profiles of the epididymal duct Efferent ducts Seminiferous tubules Rete testis tubules

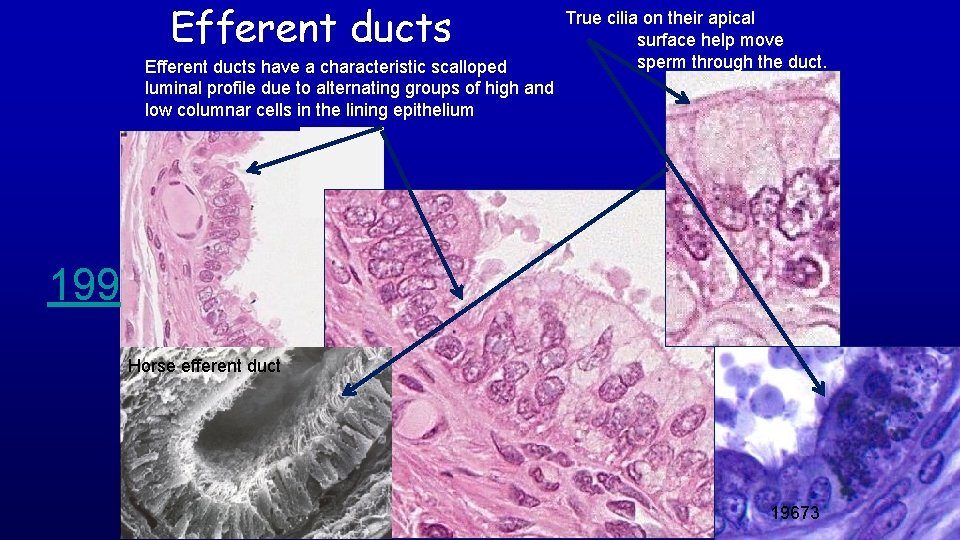
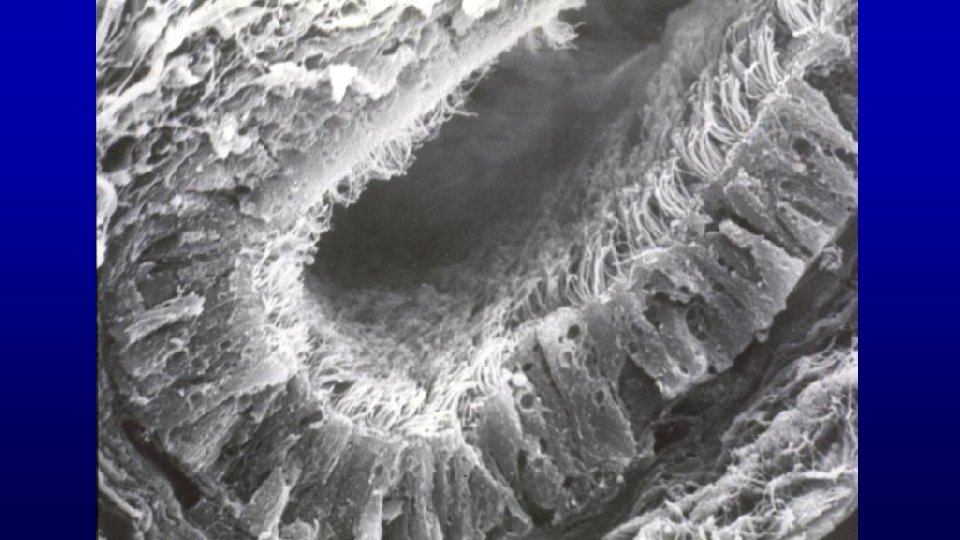
Efferent ducts have a characteristic scalloped luminal profile due to alternating groups of high and low columnar cells in the lining epithelium True cilia on their apical surface help move sperm through the duct. Lumen 199 Horse efferent duct 19673

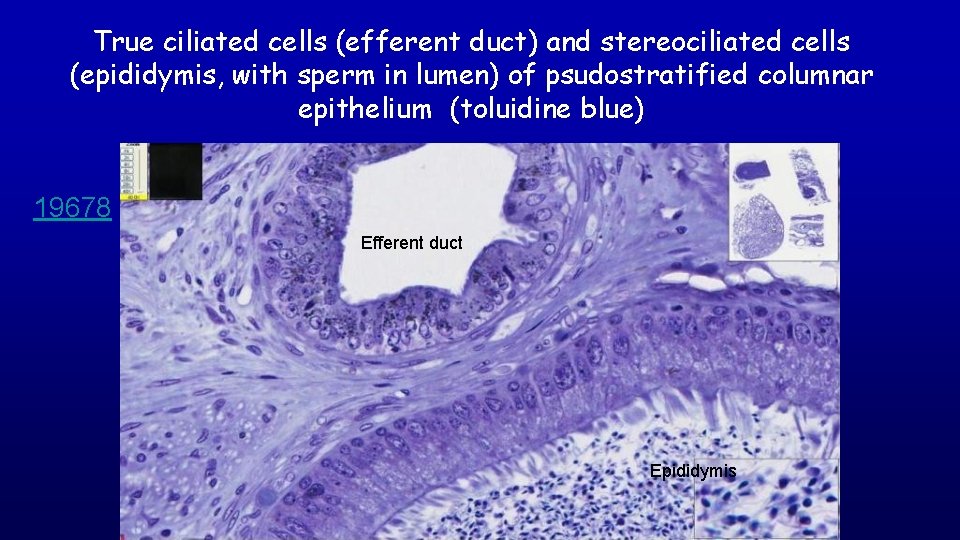
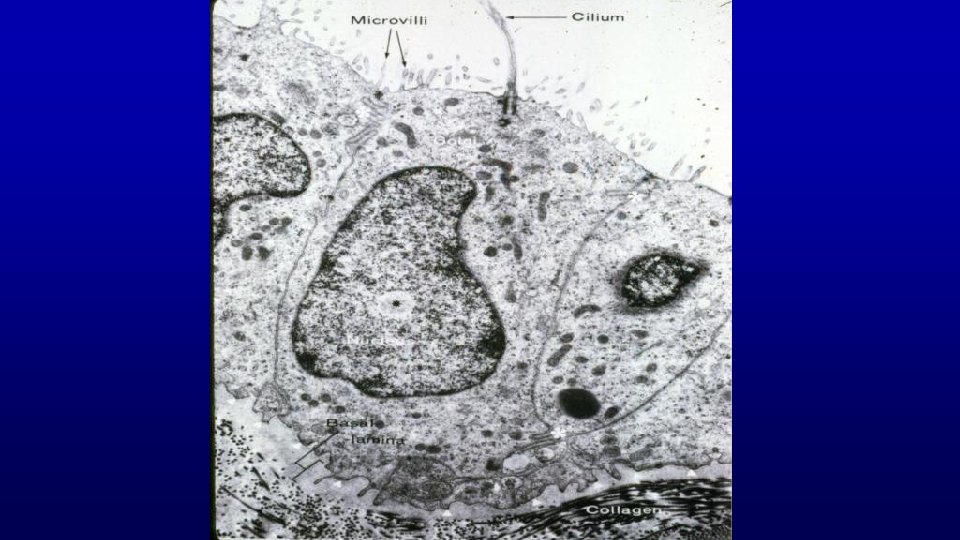
True ciliated cells (efferent duct) and stereociliated cells (epididymis, with sperm in lumen) of psudostratified columnar epithelium (toluidine blue) 19678 Efferent duct Epididymis

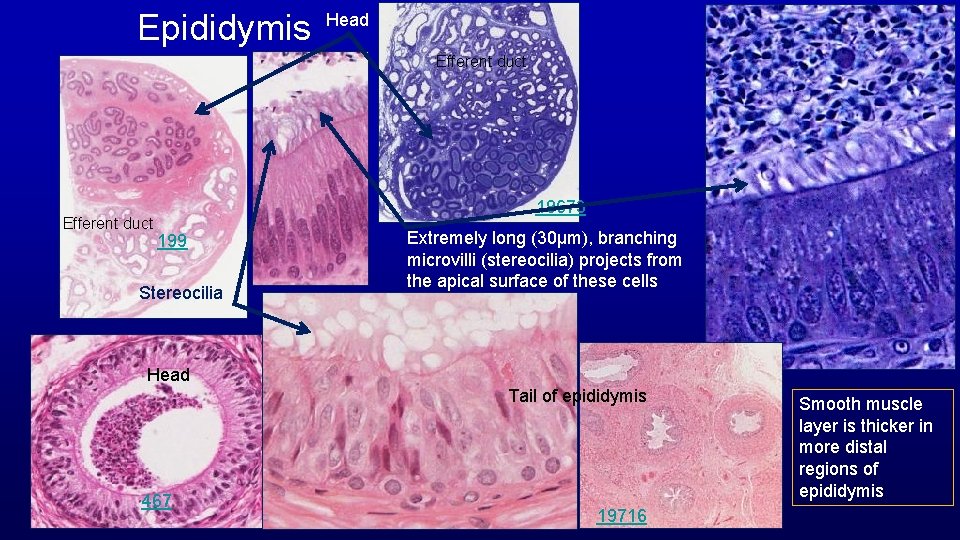
Epididymis Head Efferent duct 19673 199 Stereocilia Extremely long (30µm), branching microvilli (stereocilia) projects from the apical surface of these cells Head Tail of epididymis 467 19716 Smooth muscle layer is thicker in more distal regions of epididymis

Human







Human

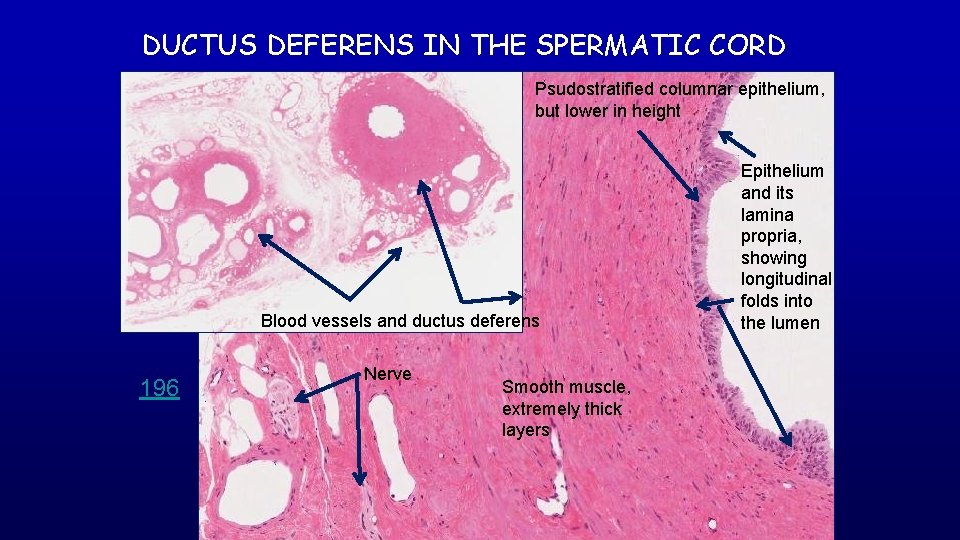
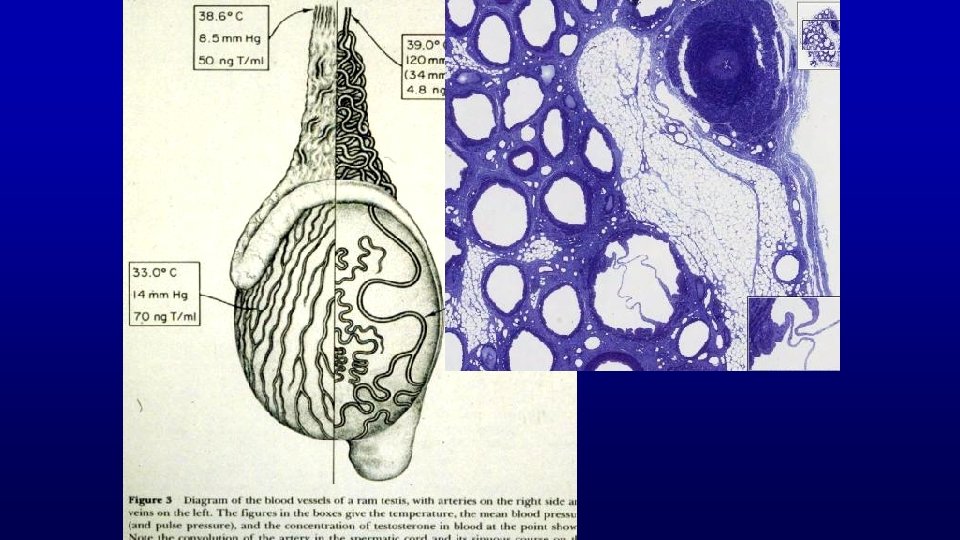
DUCTUS DEFERENS IN THE SPERMATIC CORD Psudostratified columnar epithelium, but lower in height Blood vessels and ductus deferens 196 Nerve Smooth muscle, extremely thick layers Epithelium and its lamina propria, showing longitudinal folds into the lumen

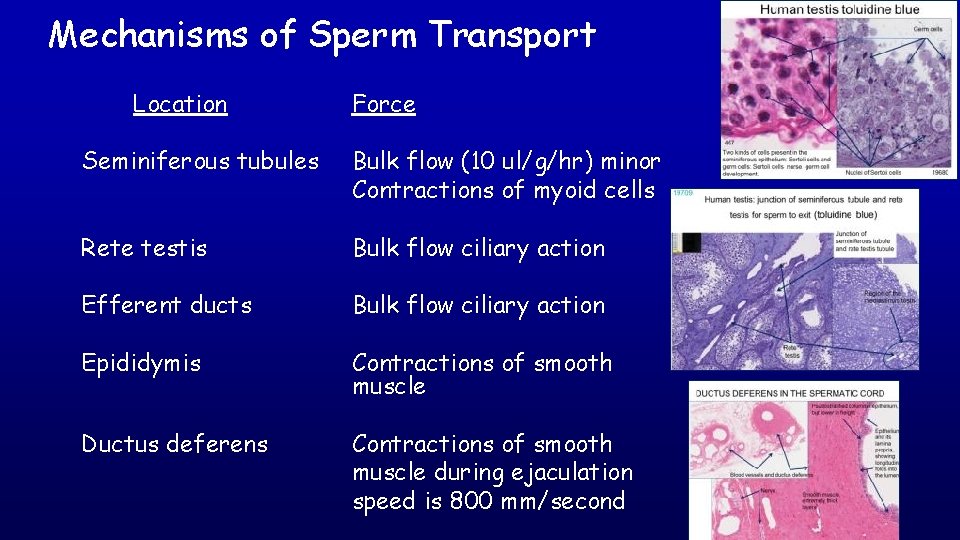
Mechanisms of Sperm Transport Location Force Seminiferous tubules Bulk flow (10 ul/g/hr) minor Contractions of myoid cells Rete testis Bulk flow ciliary action Efferent ducts Bulk flow ciliary action Epididymis Contractions of smooth muscle Ductus deferens Contractions of smooth muscle during ejaculation speed is 800 mm/second

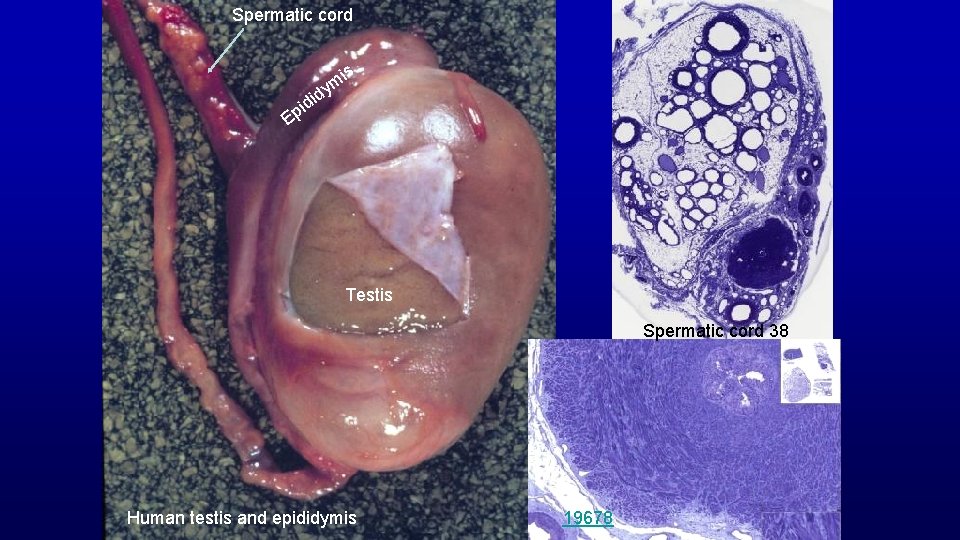
Spermatic cord is d i pid ym E Testis Spermatic cord 38 Human testis and epididymis 19678



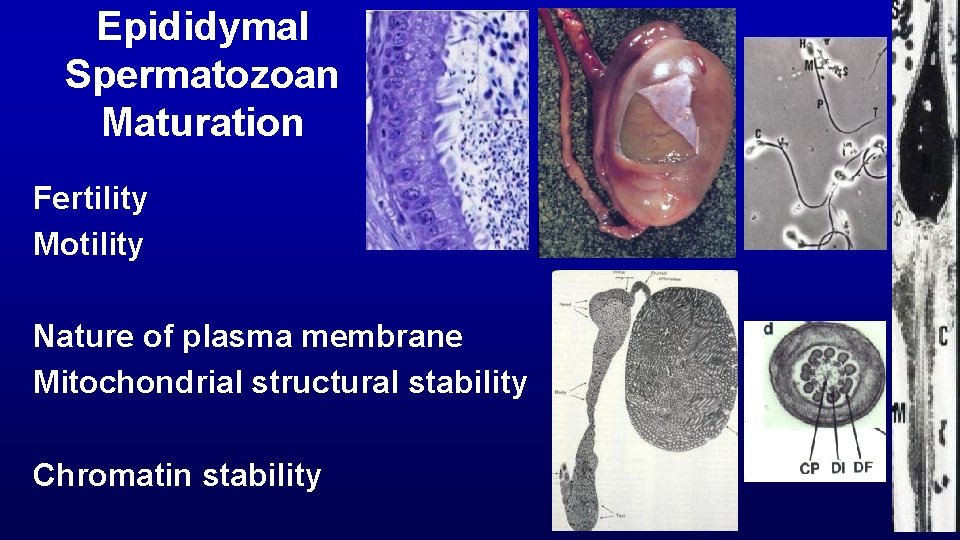
Epididymal Spermatozoan Maturation Fertility Motility Nature of plasma membrane Mitochondrial structural stability Chromatin stability


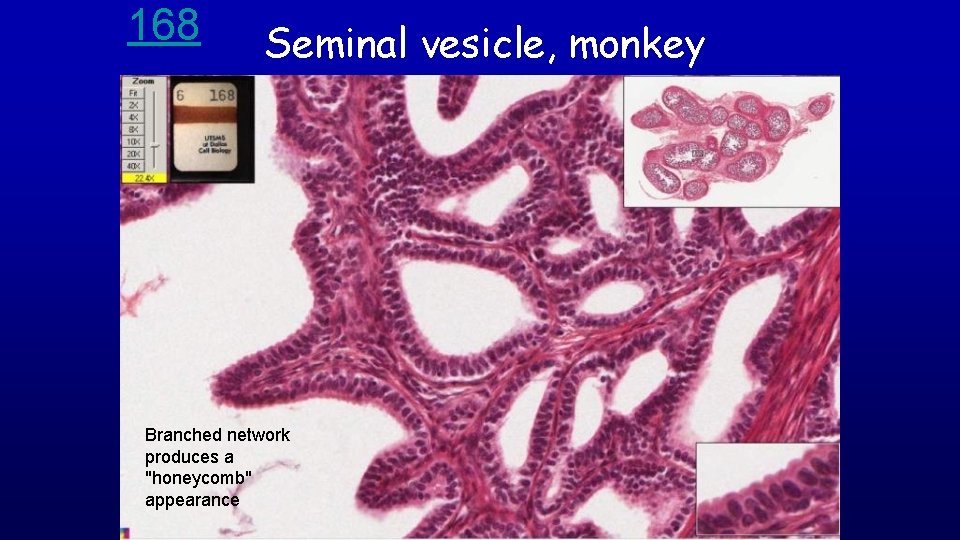
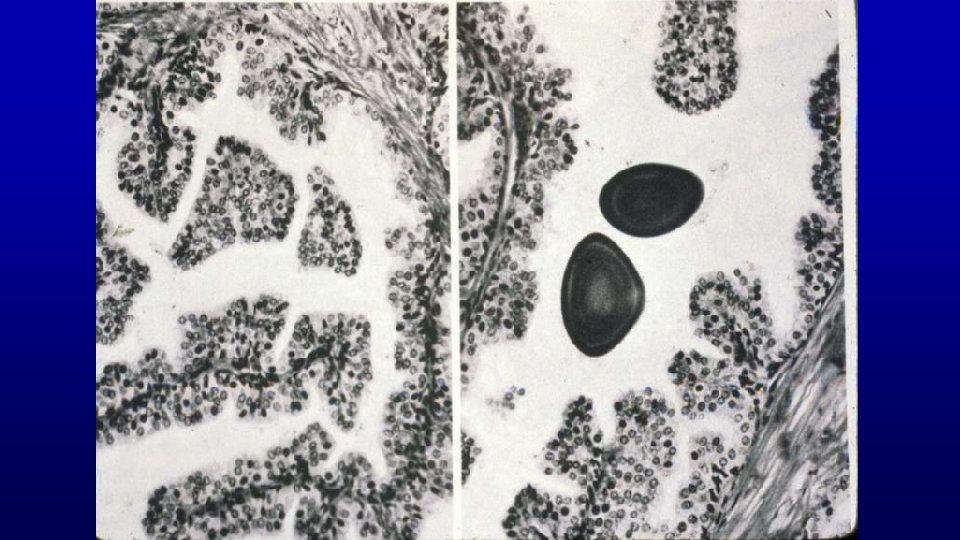
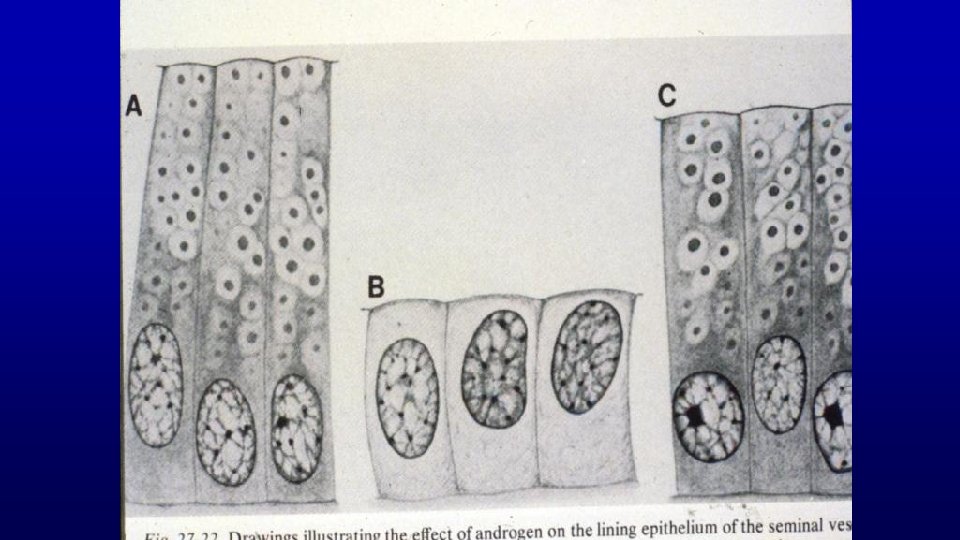
168 Seminal vesicle, monkey Branched network produces a "honeycomb" appearance



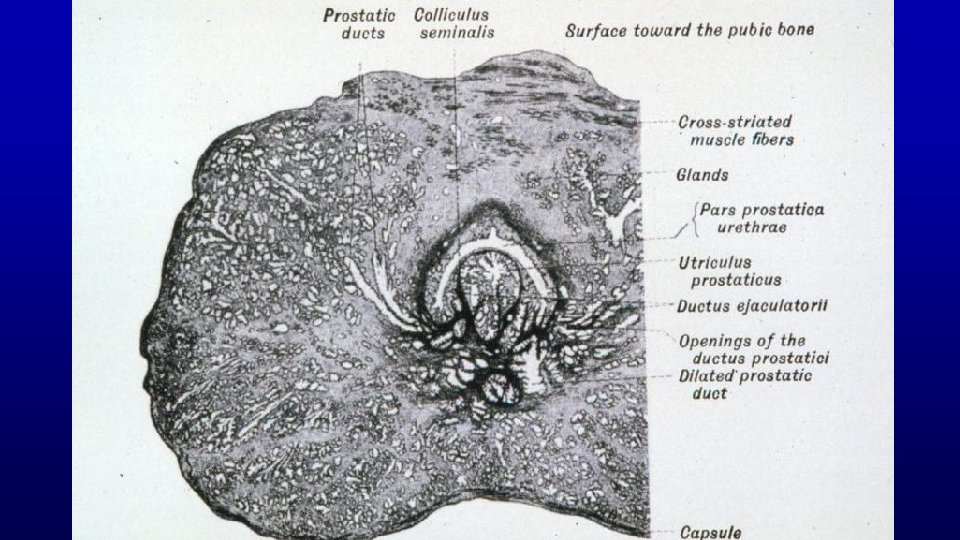
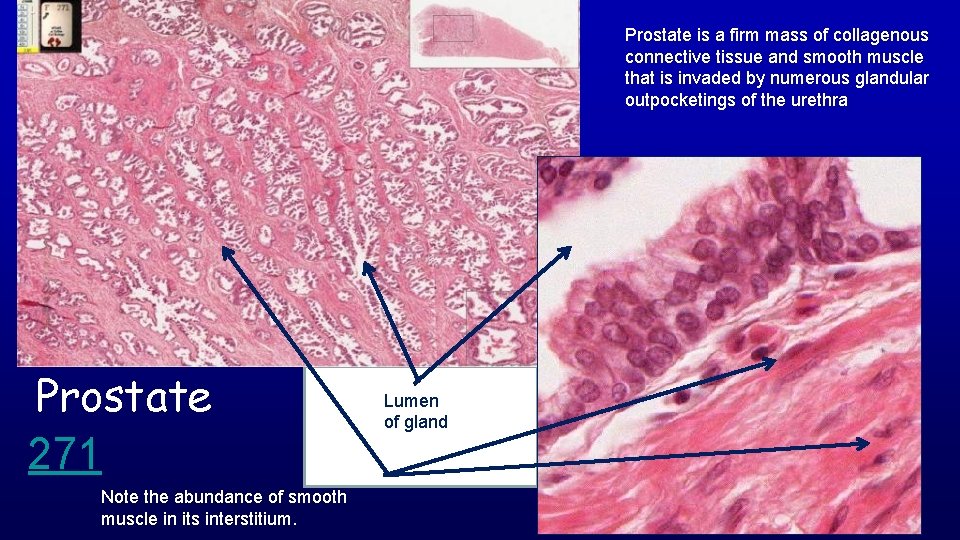
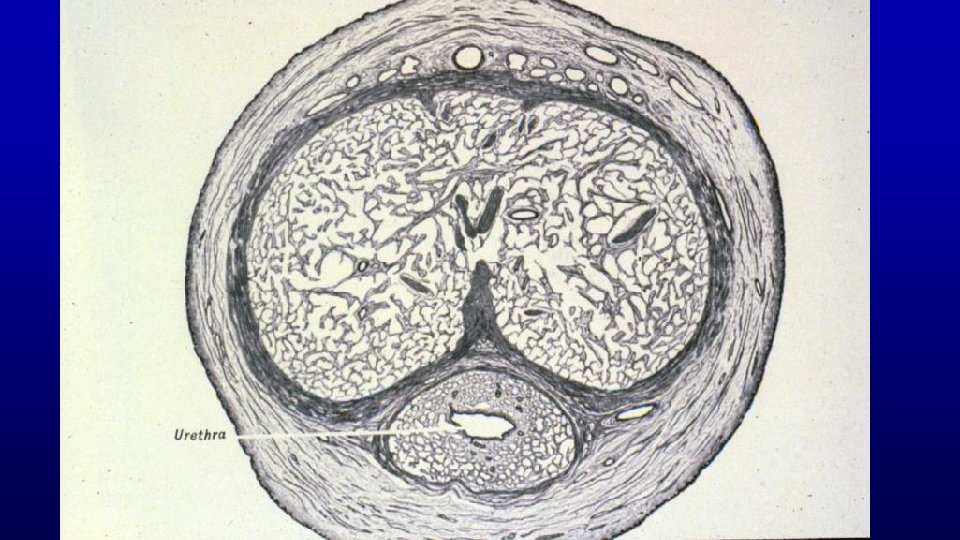
Prostate is a firm mass of collagenous connective tissue and smooth muscle that is invaded by numerous glandular outpocketings of the urethra Prostate 271 Note the abundance of smooth muscle in its interstitium. Lumen of gland

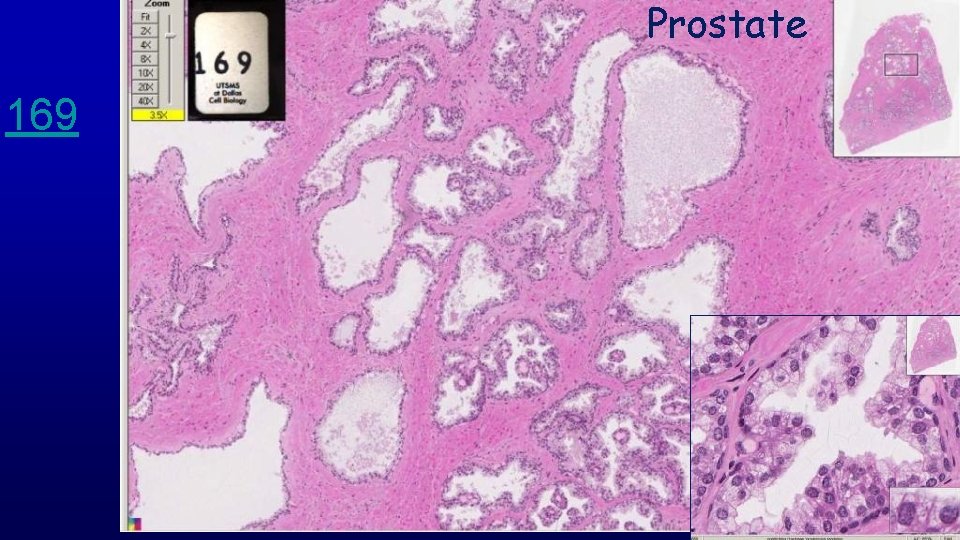
Prostate 169


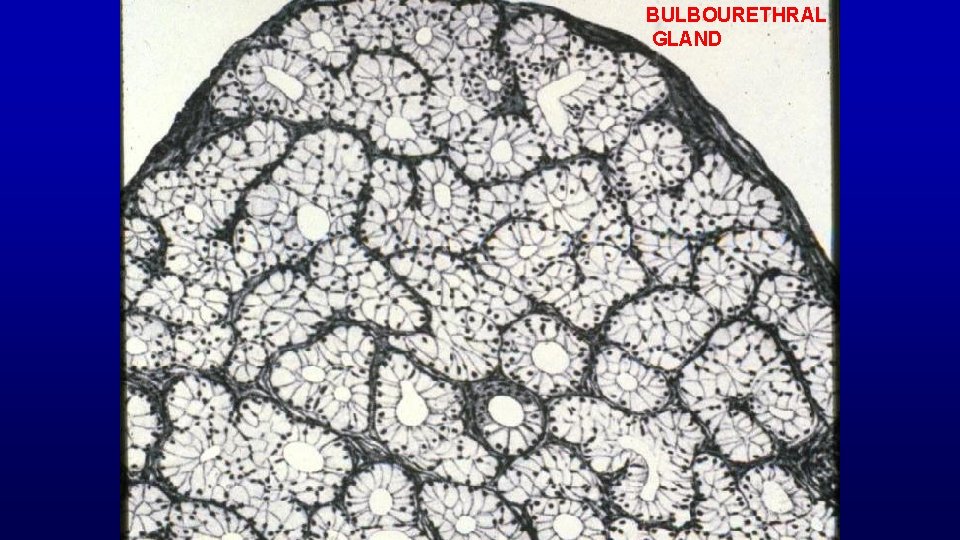
BULBOURETHRAL GLAND



Two main function of male reproductive system are to: Produce male gametes Deliver male gametes


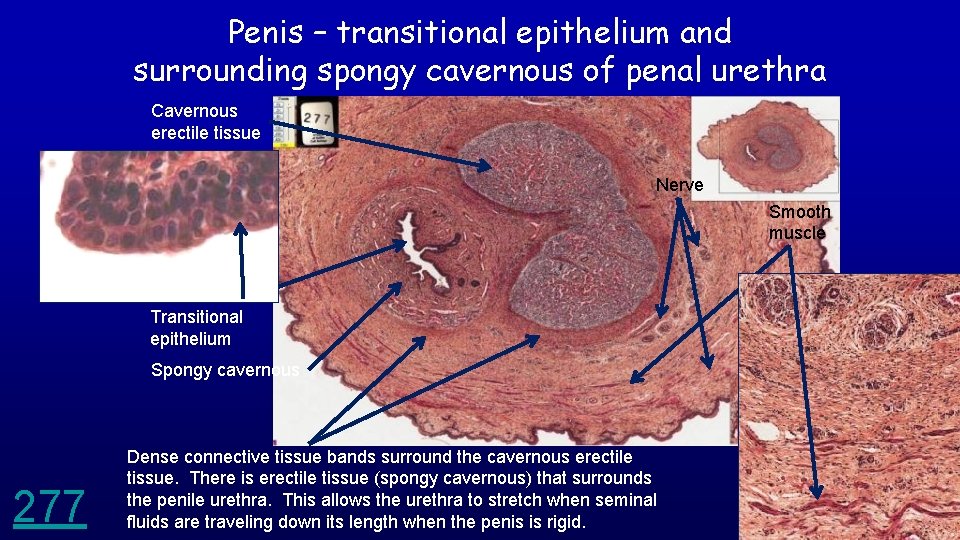
Penis – transitional epithelium and surrounding spongy cavernous of penal urethra Cavernous erectile tissue Nerve Smooth muscle Transitional epithelium Spongy cavernous 277 Dense connective tissue bands surround the cavernous erectile tissue. There is erectile tissue (spongy cavernous) that surrounds the penile urethra. This allows the urethra to stretch when seminal fluids are traveling down its length when the penis is rigid.

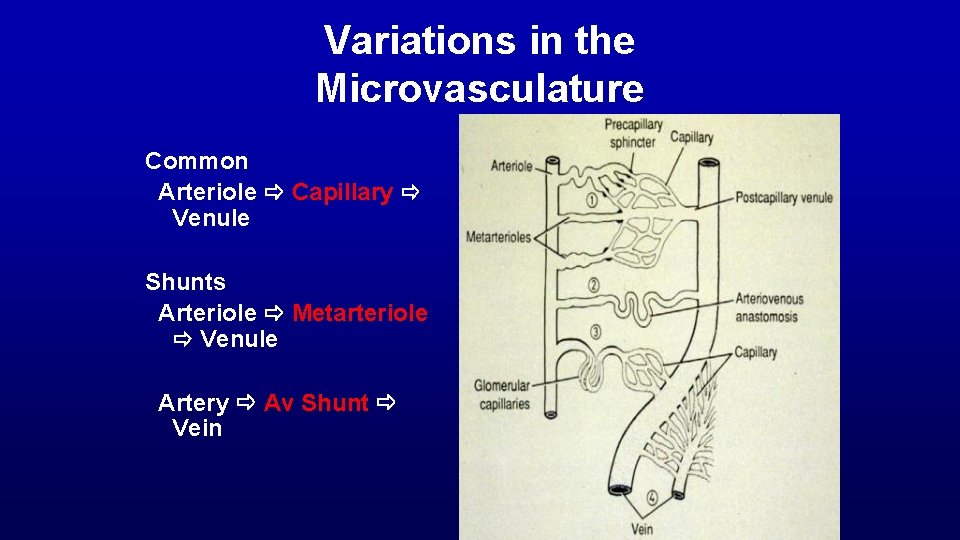
Variations in the Microvasculature Common Arteriole Capillary Venule Shunts Arteriole Metarteriole Venule Artery Av Shunt Vein

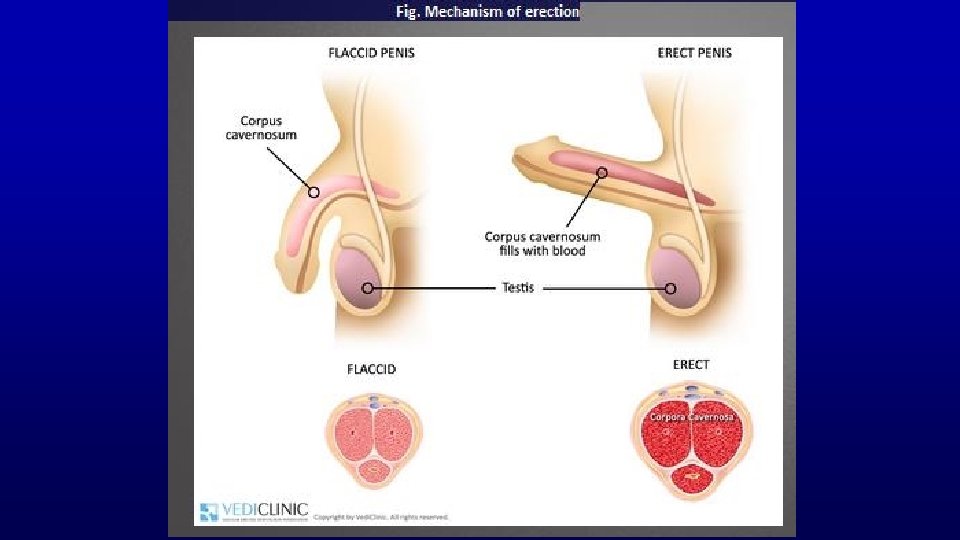
Fig. Mechanism of erection Erection occurs as a complex process, constituted by psychological, neurological, hormonal, and vascular factors. the penis is composed of three basic anatomical structures – two longitudinal cavernous bodies (a kind of chambers) and one spongy body, including the urethra. These are the cavernous bodies that (supplied by respective arteries) increase their volume during erection, owing to the inflowing blood. arteries (as opposed to veins) have their muscular layer composed of smooth muscles which – by dilation or contraction – regulate the blood flow.


Sexual arousal and increasing activity of the autonomic nervous system stimulates the release of neurotransmitters at nerve endings in the cavernous bodies or in the endothelium of the arteries. This leads to secretion of NO – nitric oxide, which is one of the strongest smooth muscle relaxants. With dilated cavernous arteries, the amount of blood flowing into the penis increases, and its outflow is hindered by a physiological compression of some specific veins. Moreover, contraction of the ischiocavernous muscle stabilises the penis in erectile position. A key factor of effective erection is the condition of the vascular system, ensuring a proper perfusion of the reproductive organs. Any pathologies of this system (e. g. , atherosclerosis, coronary disease, hypertension) lead to problems with erection.

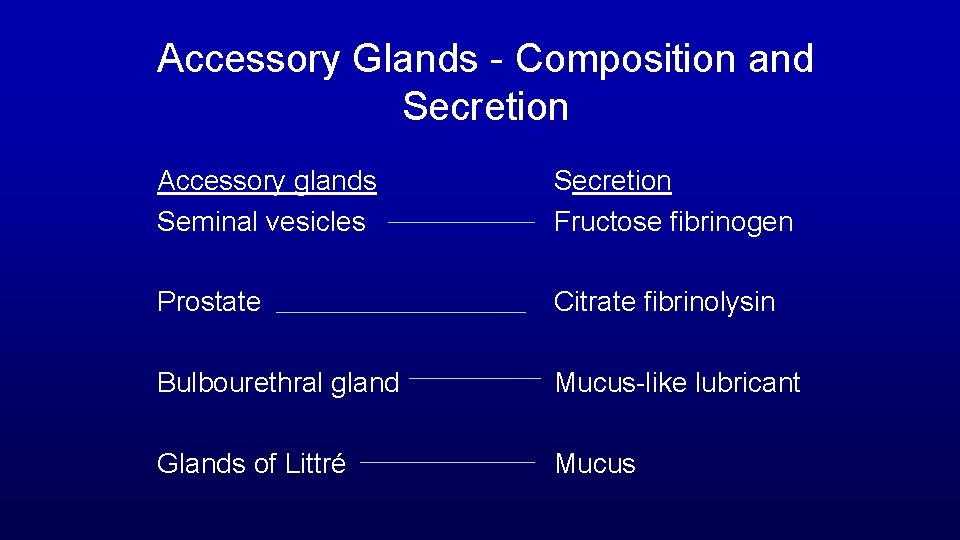
Accessory Glands - Composition and Secretion Accessory glands Seminal vesicles Secretion Fructose fibrinogen Prostate Citrate fibrinolysin Bulbourethral gland Mucus-like lubricant Glands of Littré Mucus

Functional Properties of the Accessory Glands Specific contributions of seminal plasma as measured by the split ejaculate method Fraction of Ejaculate Contains First 90% Of All Citrate 90% Of All Sperm Last 90% Of All Fructose Developmental response to androgens Source Prostate Ductus Deferens Seminal Vesicles

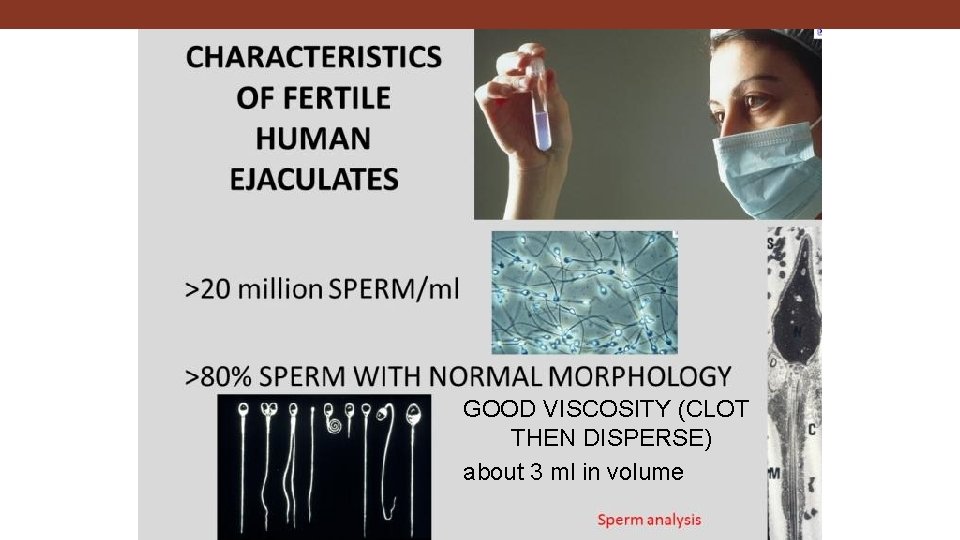
CHARACTERISTICS OF FERTILE HUMAN EJACULATES GOOD VISCOSITY (CLOT THEN DISPERSE) about 3 ml in volume




Many illustrations in these VIBS Histology You. Tube videos were modified from the following books and sources: Many thanks to original sources! Bruce Alberts, et al. 1983. Molecular Biology of the Cell. Garland Publishing, Inc. , New York, NY. Bruce Alberts, et al. 1994. Molecular Biology of the Cell. Garland Publishing, Inc. , New York, NY. William J. Banks, 1981. Applied Veterinary Histology. Williams and Wilkins, Los Angeles, CA. Hans Elias, et al. 1978. Histology and Human Microanatomy. John Wiley and Sons, New York, NY. Don W. Fawcett. 1986. Bloom and Fawcett. A textbook of histology. W. B. Saunders Company, Philadelphia, PA. Don W. Fawcett. 1994. Bloom and Fawcett. A textbook of histology. Chapman and Hall, New York, NY. Arthur W. Ham and David H. Cormack. 1979. Histology. J. S. Lippincott Company, Philadelphia, PA. Luis C. Junqueira, et al. 1983. Basic Histology. Lange Medical Publications, Los Altos, CA. L. Carlos Junqueira, et al. 1995. Basic Histology. Appleton and Lange, Norwalk, CT. L. L. Langley, et al. 1974. Dynamic Anatomy and Physiology. Mc. Graw-Hill Book Company, New York, NY. W. W. Tuttle and Byron A. Schottelius. 1969. Textbook of Physiology. The C. V. Mosby Company, St. Louis, MO. Leon Weiss. 1977. Histology Cell and Tissue Biology. Elsevier Biomedical, New York, NY. Leon Weiss and Roy O. Greep. 1977. Histology. Mc. Graw-Hill Book Company, New York, NY. Nature (http: //www. nature. com), Vol. 414: 88, 2001. A. L. Mescher 2013 Junqueira’s Basis Histology text and atlas, 13 th ed. Mc. Graw Internet images and videos on biological presentations

During the hour of this lecture the average male produced 6. 6 million spermatozoa





Spermatogenesis is Divided into 3 Main Events Event Cell Type Spermatocytogenesis Spermatogonia 27 Days Meiosis Spermatocytes 24 Days Spermiogenesis Spermatids 23 Days Combined Duration = Duration 74 Days

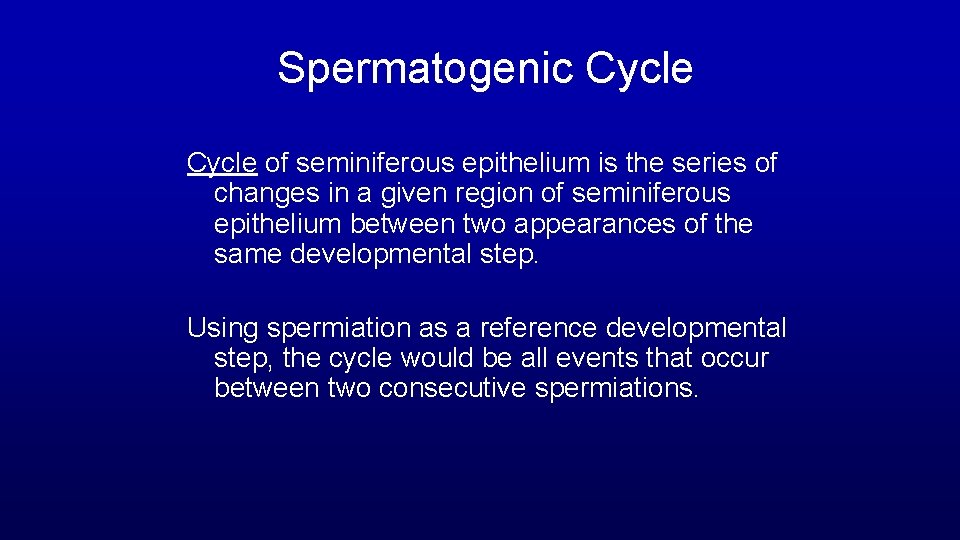
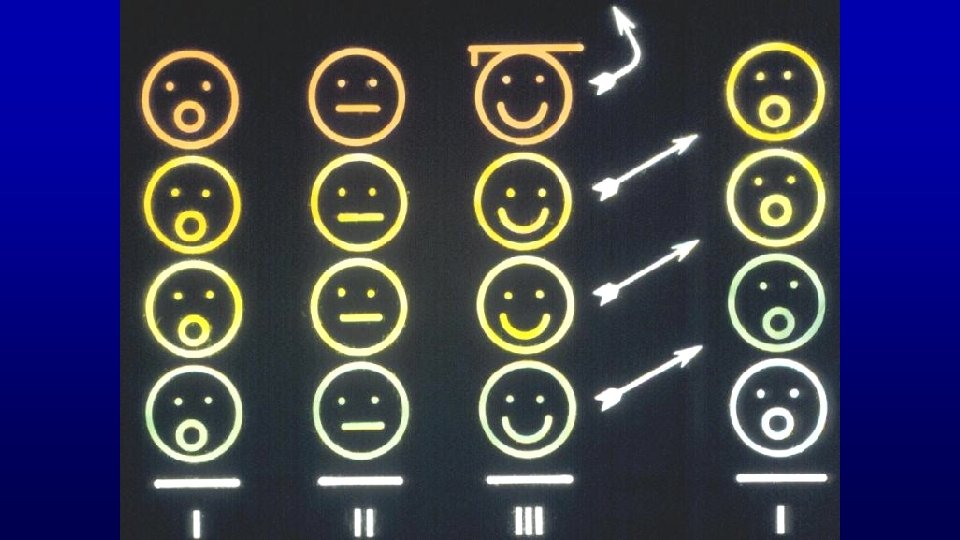
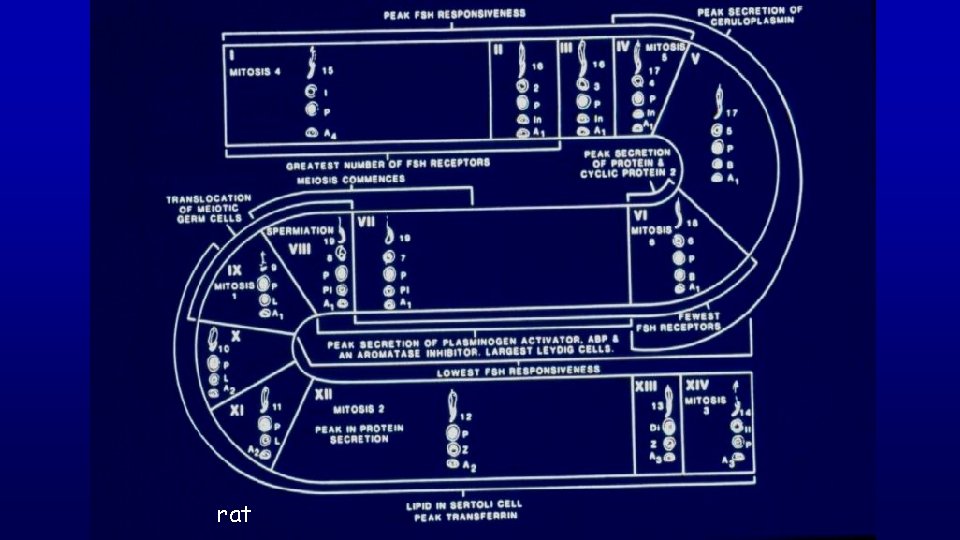
Spermatogenic Cycle of seminiferous epithelium is the series of changes in a given region of seminiferous epithelium between two appearances of the same developmental step. Using spermiation as a reference developmental step, the cycle would be all events that occur between two consecutive spermiations.


Similarities College vs. Spermatogenesis Duration of entire process is longer than the cycle length Multiple groups of participants develop simultaneously Attrition of participants reduce product yield Timing of entry of participants in different groups create defined stages of the cycle

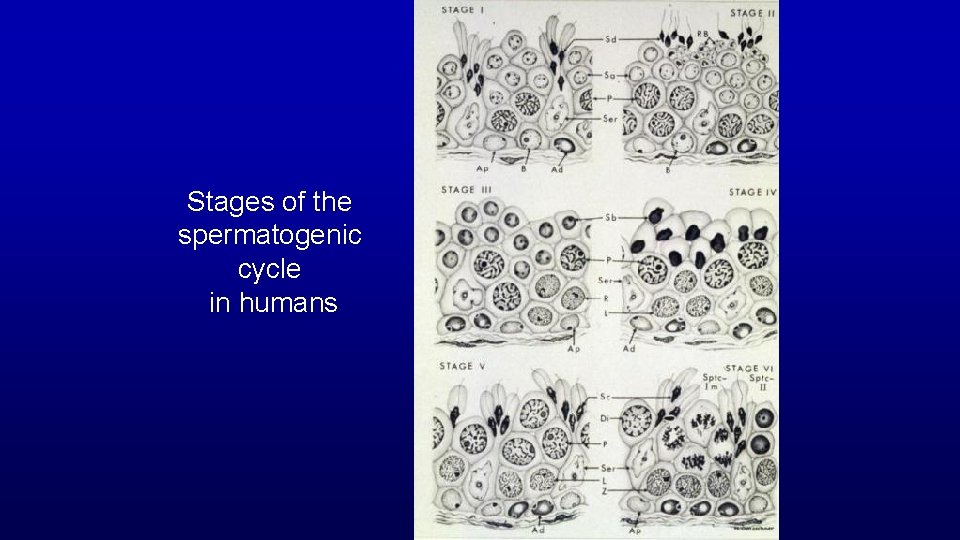
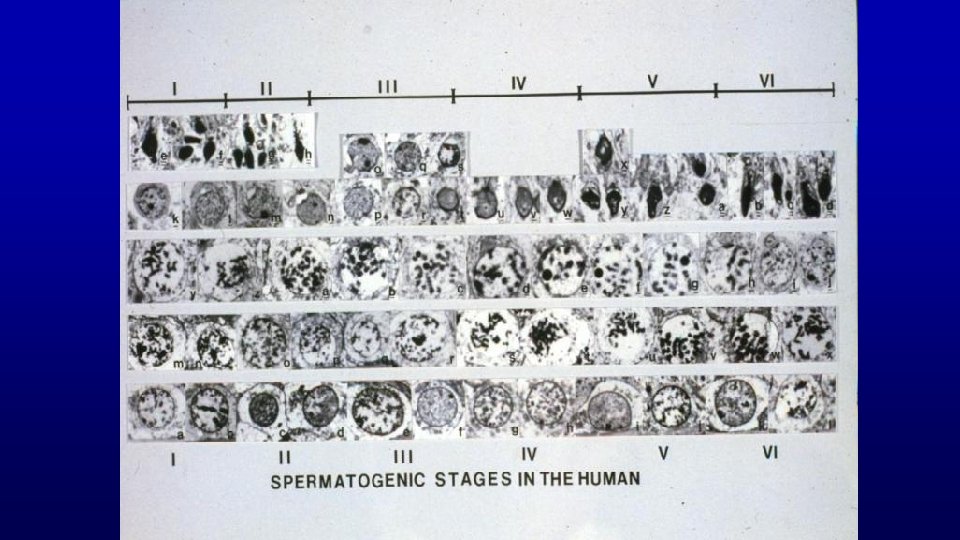
Stages of the spermatogenic cycle in humans

Human spermatogenesis: path followed through spermatocytogenesis, meiosis, and spermiogenesis as a given cell travels through five spermatogenic cycles




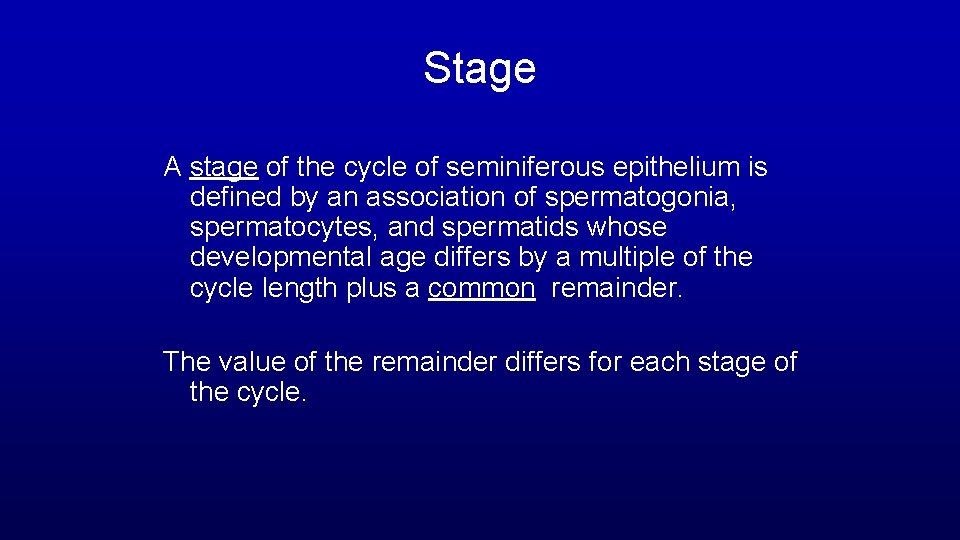
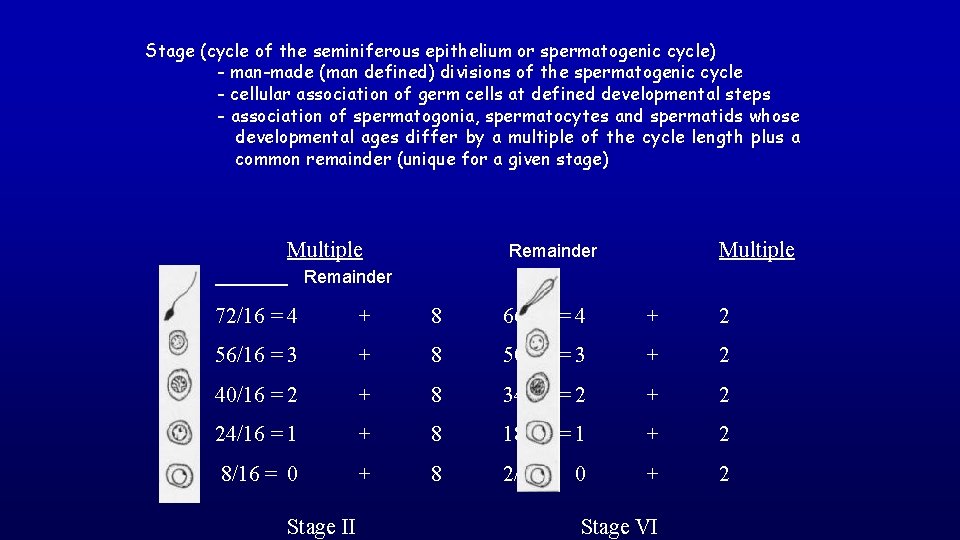
Stage A stage of the cycle of seminiferous epithelium is defined by an association of spermatogonia, spermatocytes, and spermatids whose developmental age differs by a multiple of the cycle length plus a common remainder. The value of the remainder differs for each stage of the cycle.

Stage (cycle of the seminiferous epithelium or spermatogenic cycle) - man-made (man defined) divisions of the spermatogenic cycle - cellular association of germ cells at defined developmental steps - association of spermatogonia, spermatocytes and spermatids whose developmental ages differ by a multiple of the cycle length plus a common remainder (unique for a given stage) Multiple Remainder 72/16 = 4 + 8 66/16 = 4 + 2 56/16 = 3 + 8 50/16 = 3 + 2 40/16 = 2 + 8 34/16 = 2 + 2 24/16 = 1 + 8 18/16 = 1 + 2 8/16 = 0 + 8 2/16 = 0 + 2 Stage II Stage VI



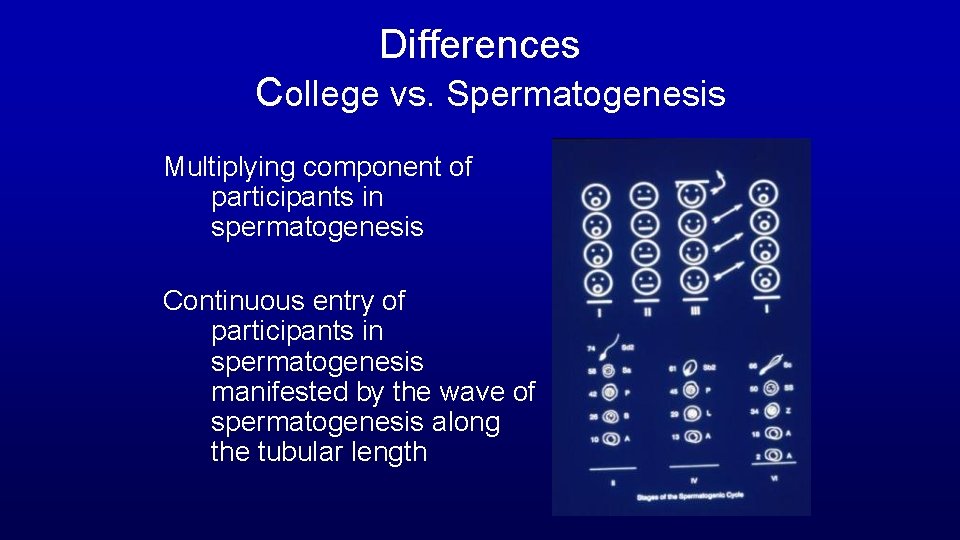
Differences College vs. Spermatogenesis Multiplying component of participants in spermatogenesis Continuous entry of participants in spermatogenesis manifested by the wave of spermatogenesis along the tubular length

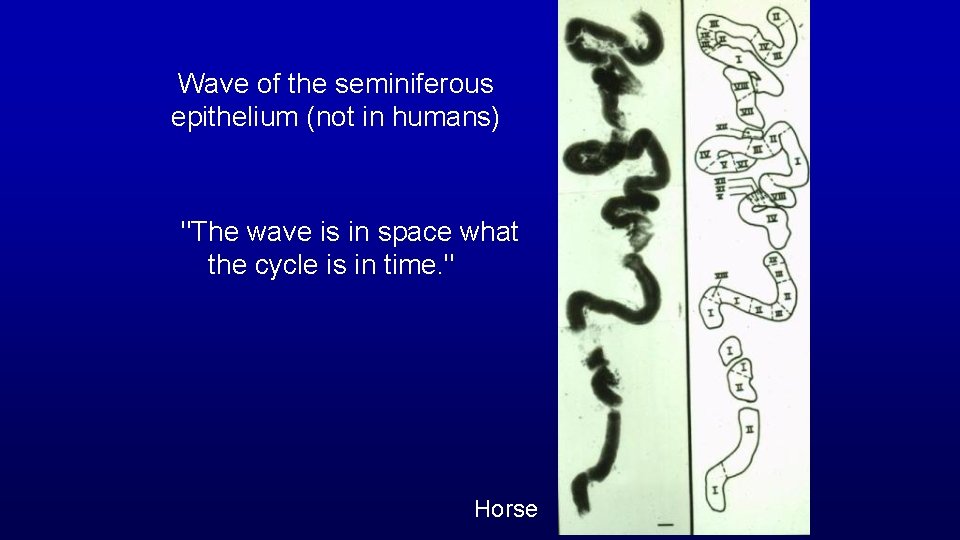
Wave of the seminiferous epithelium (not in humans) "The wave is in space what the cycle is in time. " Horse

rat
