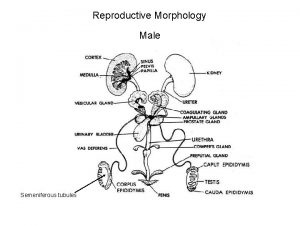
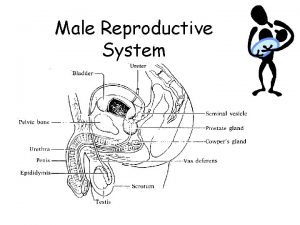
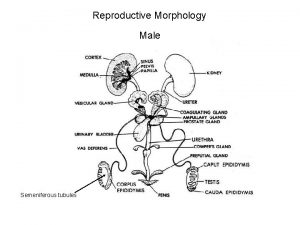
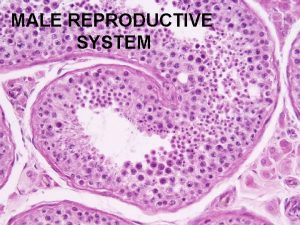
Male infertility Male infertility Reproductive medicine has undergone



















- Slides: 32

남성 불임증 Male infertility


남성불임 Male infertility Reproductive medicine has undergone tremendous changes during the 1990 s. assisted reproductive technoque (ART) With the success of these high-technologic, high-cost procedures, the evaluation of the male is often bypassed. This approach ignores the fact that many causes of male infertility, such as varicocele, ductal obstruction, and infections, are easily and effectively treated. In addition, without a full evaluation, significant diseases, such as testicular cancer, pituitary tumors, and neurologic disorders, may be overlooked

chance of a normal couple conceiving is estimated to be 20% to 25% per month, 75% by 6 months, and 90% by 1 year. approximately 15% of couples are unable to do so. Most conceptions occur when intercourse takes place within 6 days before and including the day of ovulation. Approximately 20% of cases of infertility are caused entirely by a male factor, with an additional 30% to 40% of cases involving both male and female factors. Therefore, a male factor is present in one half of infertile couples.

I. Causes of male infertility 1. Pre-testicular causes 1) Isolated gonadotropin deficiency (Kallmann's syndrome) familiar form; inherited either autosomal recessive or dominant associated with anosmia I. except gonadotropin deficiency, anterior pituitary function intact II. other congenital anomaly; congenital deafness, harelip, cleft palate III. craniofacial symmetry, renal abnormality IV. 2) Pituitary disease VI. prolactinemia, hemochromatosis VIII. 3) Exogenous or endogenous hormone IX. estrogen/androgen excess, glucocorticoid excess, X. hyper- and hypothyroidism XI.

2. Testicular causes 1) Chromosomal abnormality (1) Klinefelter's syndrome; presence of extra X chromosome 10 % of patient have mosaicism; less severe feature and may be fertile small, firm testis, decreased androgenecity, azoospermia, gynecomastia Leydig cell may appear hyperplastic, normal number gonadotropin level are elevated, particularly FSH normal or decreased testosterone level, elevated estradiol in serum (2) XX disorder (sex reversal syndrome) 2) Bilateral anorchia (vanishing testis syndrome) 3) Sertoli cell only syndrome (Germinal cell aplasia) congenital absence of germ cell, genetic defect, androgen resistance testicular biopsy reveal complete absence of germinal element azoospermia with normal virilization normal consistency but slightly smaller size, no gynecomastia normal LH, testosterone and elevated FSH

3. Post-testicular causes 1) Disorders of sperm transport 2) Disorders of sperm motility or function (1) congenital defects of sperm tail immotile-cilia syndrome Kartagener's syndrome; immotile sperm, chronic sinusitis, bronchiectasis, situs inversus, (2) maturation defect (3) immunologic defect

II. Clinical findings 1. History male reproductive history; duration of unprotected coitus, previous pregnancy female reproductive history: age, gravida/para, ovulation, corpusluteal function personal history; puberty, undescended testis, surgical, gonadotxin, sexual, medication family history; hypogonadism, cystic fibrosis endocrine history; most experts advise vaginal intercourse every 2 days, which ensures that viable sperm are present during the 12 - to 24 -hour period in which the oocyte is within the fallopian tube and is capable of being fertilized. after a febrile illness, spermatogenesis may be impaired for 1 to 3 months. in patients with abnormal semen analyses and a history of a systemic illness within 3 months of the evaluation, additional semen analysis should be obtained over a 3 - to 6 -month period to adequately assess the patient's baseline fertility status.

2. Physical examination 1) Eunuchoid skeletal proportion Upper-body: lower-body ratio <1 arm span 2 inches greater than height 2) Lack of adult male hair distribution sparse axillary, pubic, facial, body hair lack of recession of hair on temporal bone 3) Infantile genitalia small penis, testes, and prostate, underdeveloped scrotum 4) diminished muscular development and mass

3. Laboratory studies 1) Semen analysis highly predictive of significance of male factor specimen collected in clean, wide mouthed container or in special condom should be examined within 2 hours standard period of abstinence is 2 - 3 days azoospermia: qualitative test for fructose (1) Low ejaculate volume, lack of fructose, failure of coagulation: a. seminal vesicle absence b. absence of vas deferens c. atresia ejaculatory duct

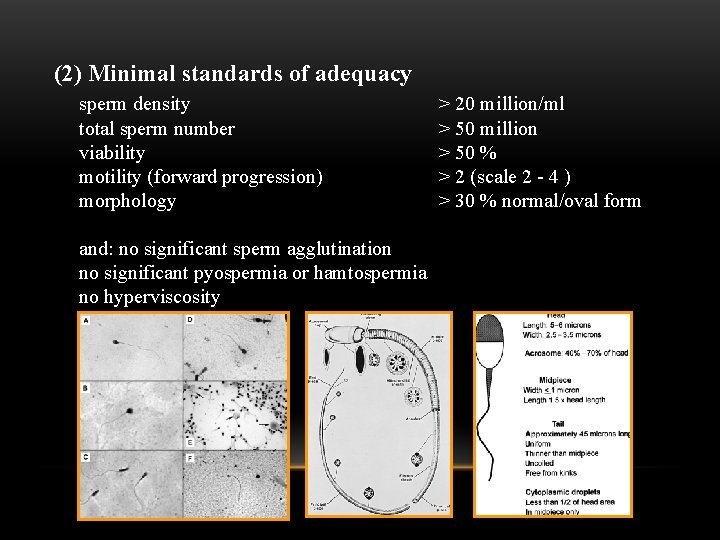
(2) Minimal standards of adequacy sperm density total sperm number viability motility (forward progression) morphology and: no significant sperm agglutination no significant pyospermia or hamtospermia no hyperviscosity > 20 million/ml > 50 million > 50 % > 2 (scale 2 - 4 ) > 30 % normal/oval form


Figure 19 -3 Algorithm for the evaluation of the patient with low-volume or absent (aspermia) ejaculate. AIH, artificial insemination using husband's sperm; TURED, transurethral resection of the ejaculatory ducts

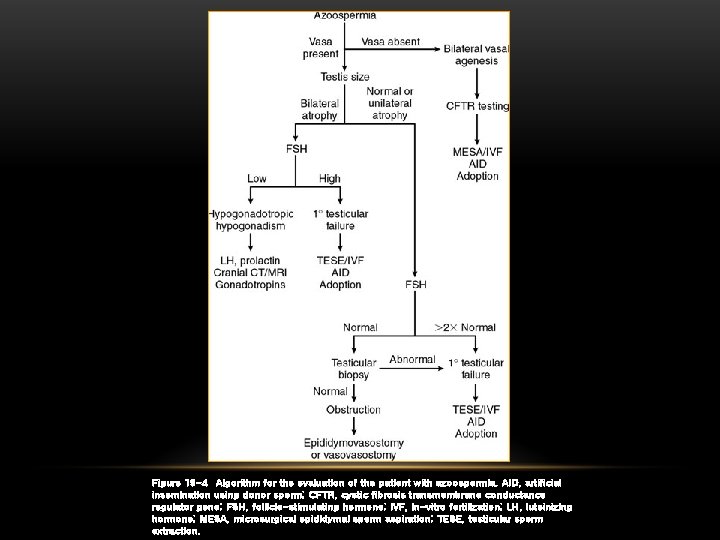
Figure 19 -4 Algorithm for the evaluation of the patient with azoospermia. AID, artificial insemination using donor sperm; CFTR, cystic fibrosis transmembrane conductance regulator gene; FSH, follicle-stimulating hormone; IVF, in-vitro fertilization; LH, luteinizing hormone; MESA, microsurgical epididymal sperm aspiration; TESE, testicular sperm extraction.

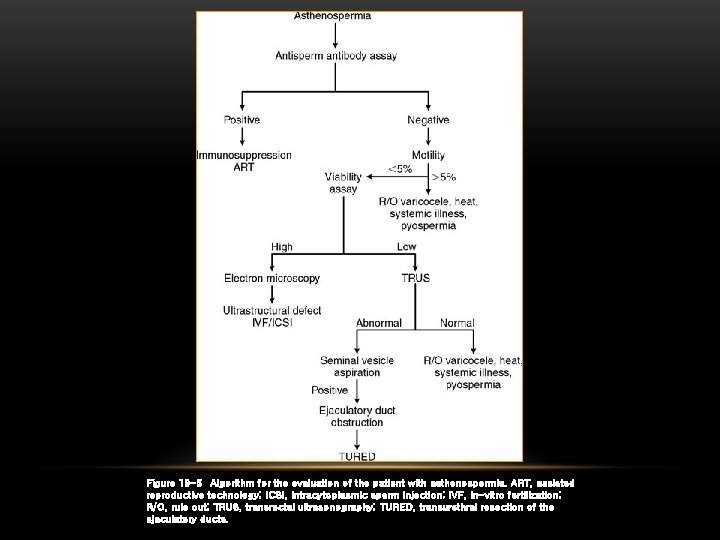
Figure 19 -5 Algorithm for the evaluation of the patient with asthenospermia. ART, assisted reproductive technology; ICSI, intracytoplasmic sperm injection; IVF, in-vitro fertilization; R/O, rule out; TRUS, transrectal ultrasonography; TURED, transurethral resection of the ejaculatory ducts.

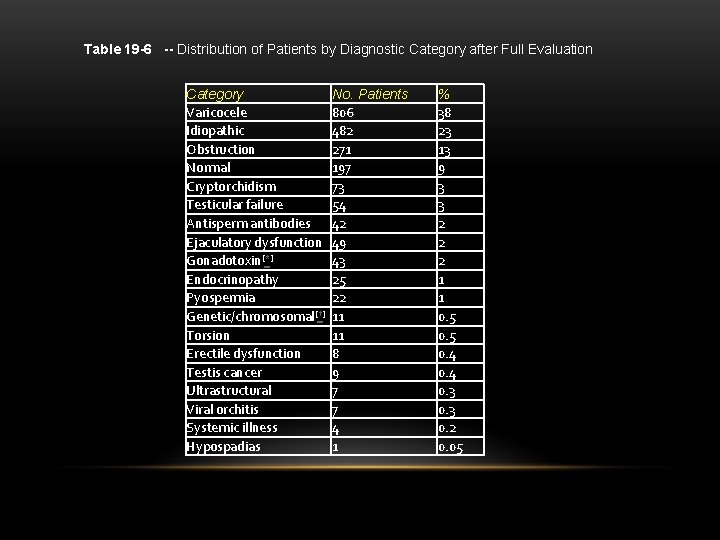
Table 19 -6 -- Distribution of Patients by Diagnostic Category after Full Evaluation Category Varicocele Idiopathic Obstruction Normal Cryptorchidism Testicular failure Antisperm antibodies Ejaculatory dysfunction Gonadotoxin[*] Endocrinopathy Pyospermia Genetic/chromosomal[†] Torsion Erectile dysfunction Testis cancer Ultrastructural Viral orchitis Systemic illness Hypospadias No. Patients 806 482 271 197 73 54 42 49 43 25 22 11 11 8 9 7 7 4 1 % 38 23 13 9 3 3 2 2 2 1 1 0. 5 0. 4 0. 3 0. 2 0. 05

(3) Computer-assisted semen analysis(CASA) new technique for semen analysis VCL (curvilinear velocity): average distance per unit time between successive position sperm along its path VSL (straight line velocity): distance between first and last position on path divided by total elapsed time LIN (linearity): VSL/VCL, measure of straightness of swimming ALH (amplitude of lateral head displacement): average perpendicular distance of lateral position of sperm head with respect to average swimming path

(4) Retrograde ejaculation confirm by reduced semen volume and presence of many sperm in post ejaculation urine specimen. a. TUR or open surgical resection of bladder or prostate b. bilateral sympathectomy c. bilateral retroperitoneal lymphadenectomy d. extensive pelvic surgery; proctectomy and colectomy e. diabetic visceral neuropathy f. antihypertensive drug; block sympathetic tone

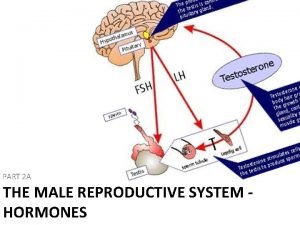
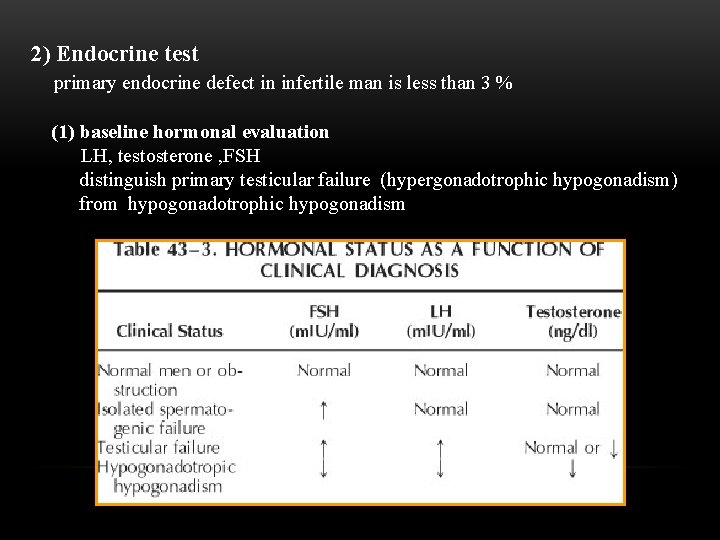
2) Endocrine test primary endocrine defect in infertile man is less than 3 % (1) baseline hormonal evaluation LH, testosterone , FSH distinguish primary testicular failure (hypergonadotrophic hypogonadism) from hypogonadotrophic hypogonadism

2) dynamic hormonal testing single pulse dose is inadequate a. h. CG test b. Gn. RH test differentiation hypogonadotrophic hypogonadism or pituitary origin from hypothalamus origin 500 mg of Gn. RH given daily for 3 days serum LH should be measured before and 60 min after last Gn. RH 3) Chromosomal study

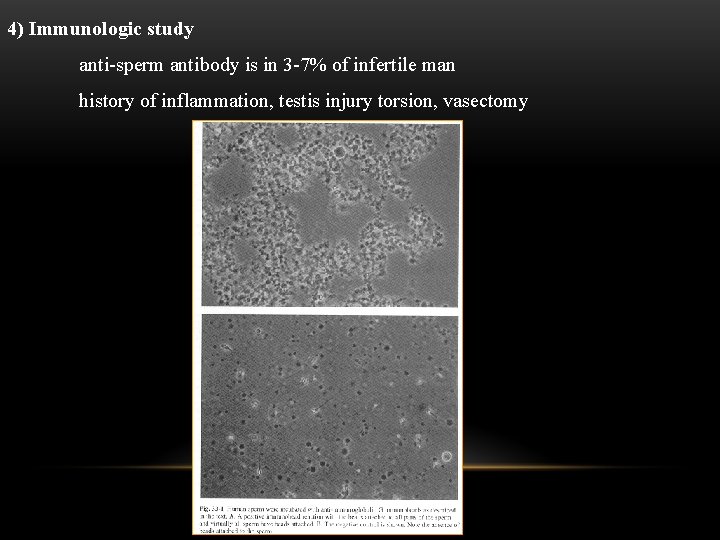
4) Immunologic study anti-sperm antibody is in 3 -7% of infertile man history of inflammation, testis injury torsion, vasectomy

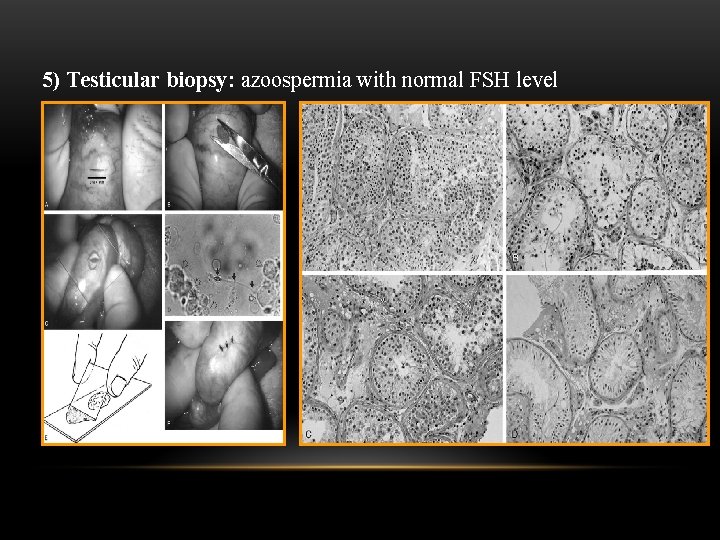
5) Testicular biopsy: azoospermia with normal FSH level

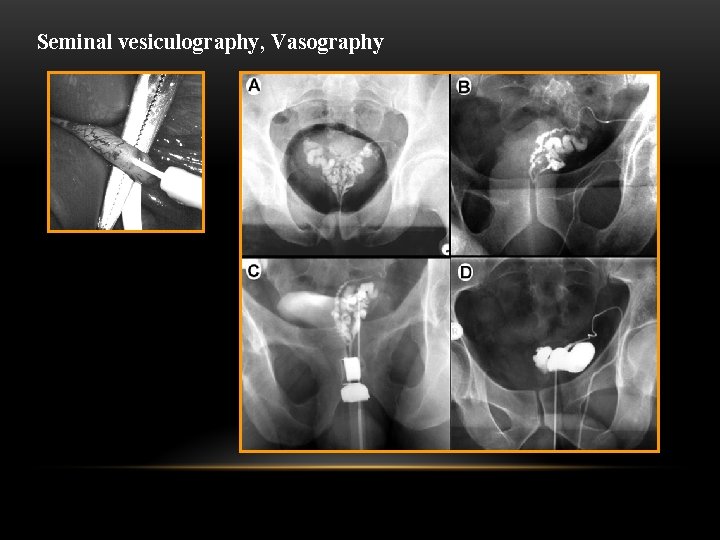
Seminal vesiculography, Vasography

6) Sperm function test (1) postcoital test (2) sperm penetration assay (3) hemizona assay (4) hyposmotic swelling test (HOS) (5) hyperactivity test

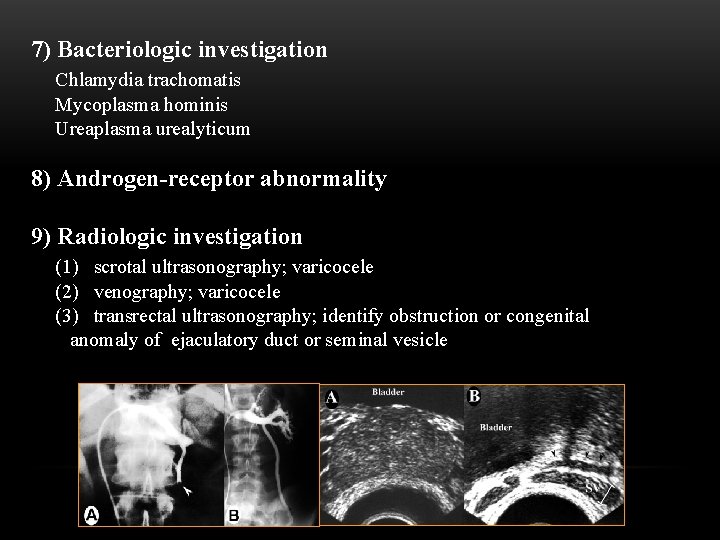
7) Bacteriologic investigation Chlamydia trachomatis Mycoplasma hominis Ureaplasma urealyticum 8) Androgen-receptor abnormality 9) Radiologic investigation (1) scrotal ultrasonography; varicocele (2) venography; varicocele (3) transrectal ultrasonography; identify obstruction or congenital anomaly of ejaculatory duct or seminal vesicle

Goals of evaluation of infertile male are to identify (1) reversible conditions; (2) irreversible causes that may be managed by ARTs using the male partner's sperm; (3) irreversible conditions that may not be managed by these techniques and in which the couple should be advised to pursue donor insemination or adoption; (4) significant underlying medical pathology; (5) genetic and/or chromosomal abnormalities that may affect either the patient or his offspring.

III. Differential diagnosis of male infertility 1. Treatable causes 1) varicocle 2) obstruction (acquired /congenital) 3) infection 4) ejaculatory dysfunction 5) hypogonadotrotic hypogonadism 6) immunologic problem 7) sexual dysfunction 8) hyperprolactinemia 2. Potentially treatable causes 1) idiopathic 2) cryptorchidism 3) vasal agenesis 4) gonadotoxin (drug, radiation) 3. Untreatable causes 1) bilateral anorchia 2) germinal cell aplasia 3) primary testicular failure 4) chromosomal anomaly 5) immotile cilia syndrome

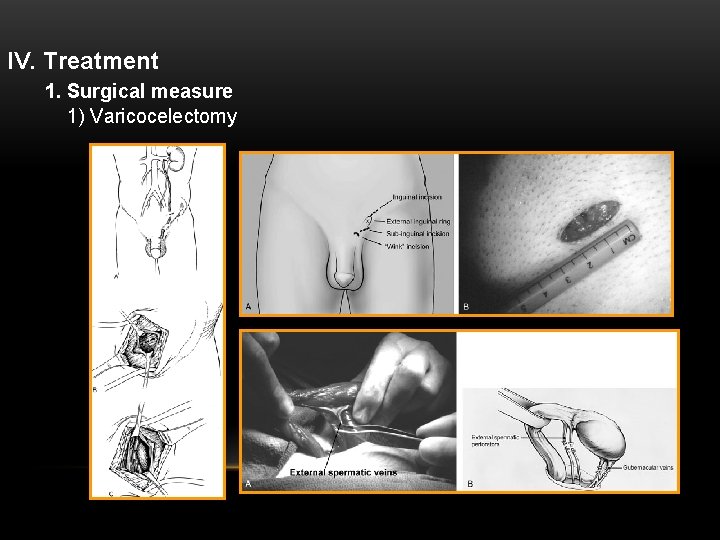
IV. Treatment 1. Surgical measure 1) Varicocelectomy

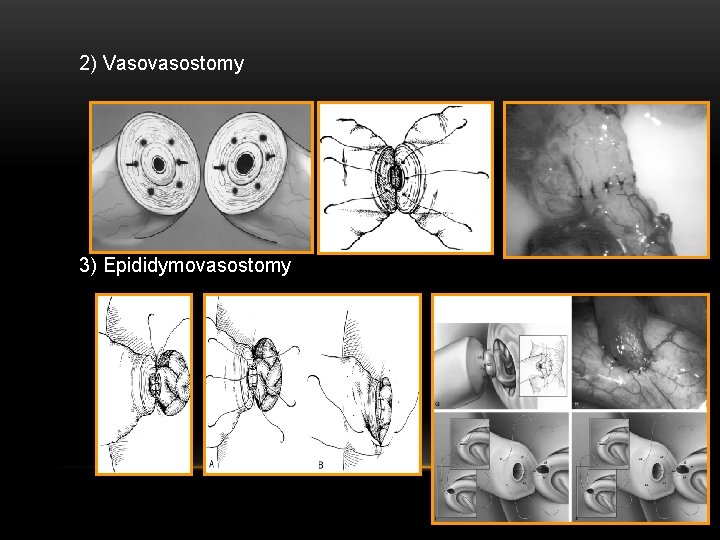
2) Vasovasostomy 3) Epididymovasostomy

4) Transurethral resection of ejaculatory duct 5) microsurgical epididymal sperm aspiration (MESA) 6) prophylactic surgical measure (1) orchiopexy (2) operation of testicular torsion (3) electroejaculation

2. Medical measure 1) Endocrine therapy hypogonadotropic hypogonadism: h. CG, h. MG Gn. RH 2) Therapy for immunologic infertility corticosteroid 3) Therapy for retrograde ejaculation alpha sympathomimetic innervation 4) Treatment of infection 5) Empirical therapy a. antiestorgen; clomiphene b. historical therapy

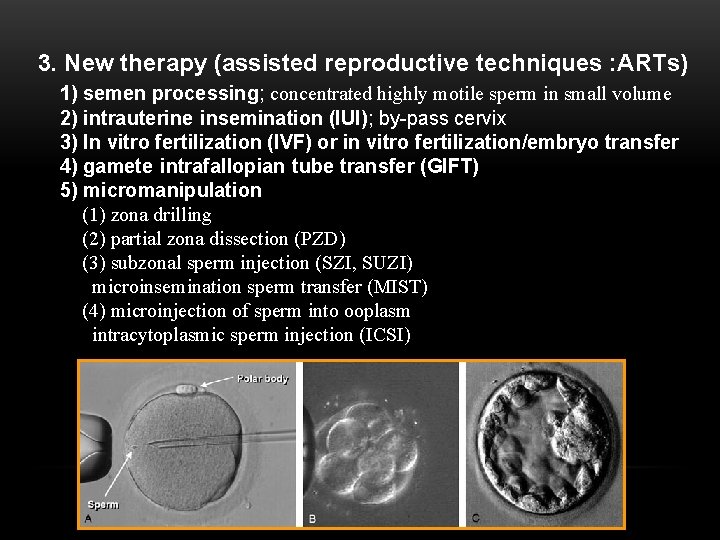
3. New therapy (assisted reproductive techniques : ARTs) 1) semen processing; concentrated highly motile sperm in small volume 2) intrauterine insemination (IUI); by-pass cervix 3) In vitro fertilization (IVF) or in vitro fertilization/embryo transfer 4) gamete intrafallopian tube transfer (GIFT) 5) micromanipulation (1) zona drilling (2) partial zona dissection (PZD) (3) subzonal sperm injection (SZI, SUZI) microinsemination sperm transfer (MIST) (4) microinjection of sperm into ooplasm intracytoplasmic sperm injection (ICSI)
 Hijama points
Hijama points Cystic fibrosis male infertility
Cystic fibrosis male infertility Forms of chicken
Forms of chicken Diploma in reproductive medicine
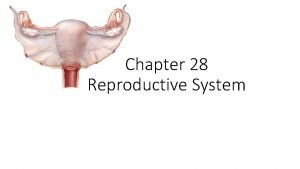
Diploma in reproductive medicine Parts of male and female reproductive system
Parts of male and female reproductive system Differences between male and female reproductive organ
Differences between male and female reproductive organ Pearson
Pearson In pila fertilization is
In pila fertilization is What are primary sexual characteristics
What are primary sexual characteristics Male reproductive system information
Male reproductive system information Unit 6 human development lesson 1 pregnancy
Unit 6 human development lesson 1 pregnancy Male reproductive system
Male reproductive system Art-labeling activity: the male reproductive system, part 1
Art-labeling activity: the male reproductive system, part 1 Plants reproductive system
Plants reproductive system Base of prostate gland
Base of prostate gland Exercise 42 anatomy of the reproductive system
Exercise 42 anatomy of the reproductive system Male genitalia
Male genitalia Asexual reproduction
Asexual reproduction Where is pollen produced
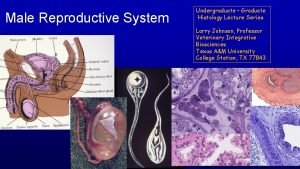
Where is pollen produced Figure 28-1 the male reproductive system
Figure 28-1 the male reproductive system Note on male reproductive system
Note on male reproductive system Figure 16-1 is a sagittal view of the male reproductive
Figure 16-1 is a sagittal view of the male reproductive Male reproductive system labeled
Male reproductive system labeled Bladder fetal pig
Bladder fetal pig Male reproductive system from front
Male reproductive system from front Where is the sperm located
Where is the sperm located Male reproductive system
Male reproductive system Figure 16-5 is a sagittal view of the female
Figure 16-5 is a sagittal view of the female Uncut penis
Uncut penis Reproductive system summary
Reproductive system summary Lesson 20.2 the male reproductive system
Lesson 20.2 the male reproductive system Oogenesis process
Oogenesis process Colon function in male reproductive system
Colon function in male reproductive system