Making Solutions Solutions and Mixtures n How are








- Slides: 10

+ Making Solutions!

+ Solutions and Mixtures n How are mixtures and solutions different? n In a mixture, you can see both substances without using a microscope n Solutions are groups of molecules that are mixed up in a completely even distribution n In a Solution, you cannot tell there is more than one substance n Scientists say that solutions are homogenous systems.

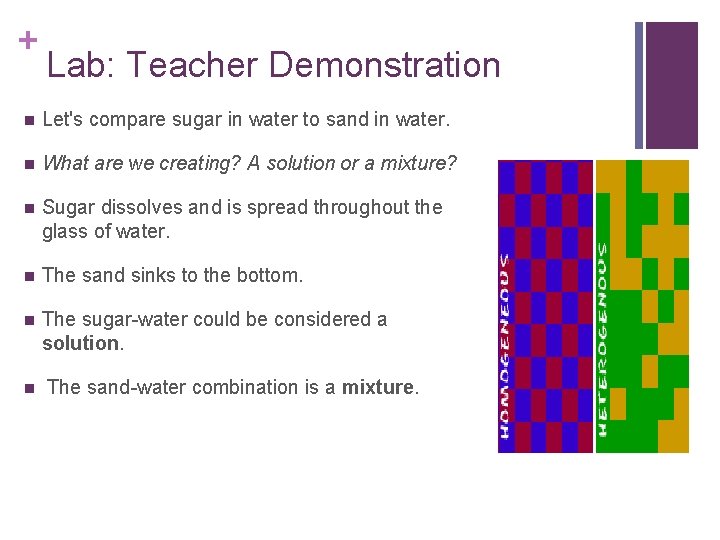
+ Lab: Teacher Demonstration n Let's compare sugar in water to sand in water. n What are we creating? A solution or a mixture? n Sugar dissolves and is spread throughout the glass of water. n The sand sinks to the bottom. n The sugar-water could be considered a solution. n The sand-water combination is a mixture.

+ Lab: Will it dissolve? n Our next lab will test whether we are creating solutions or mixtures. n In order for a solution to be created, what needs to happen to the solute? n The solute must dissolve into the solvent in order for a solution to be created.

+ Solutions and Mixtures n Everything in a solution is evenly spread out and completely mixed together. n What if we are mixing 250 m. L of water and only 5 m. L of sugar? Is this still a solution even if the parts mixed together are not equal? Talk about this with your table group! n The answer is YES! n If there is a difference in the amount of solute added to the solvent, then solution is said to have a different concentration. n Other types of mixtures can have a little more of one thing (higher concentration) on one side of the liquid when compared to the other side.

+ n Lab: Teacher Demonstration Lets mix… n n 5 m. L of sugar with 250 m. L of water 10 m. L of sugar with 250 m. L of water Are these both solutions? Yes or No Are these solutions the same? Yes or No What is different between these solutions? If there is a difference in the amount of solute added to the solvent, then solution is said to have a different concentration. n Other types of mixtures can have a little more of one thing (higher concentration) on one side of the liquid when compared to the other side.

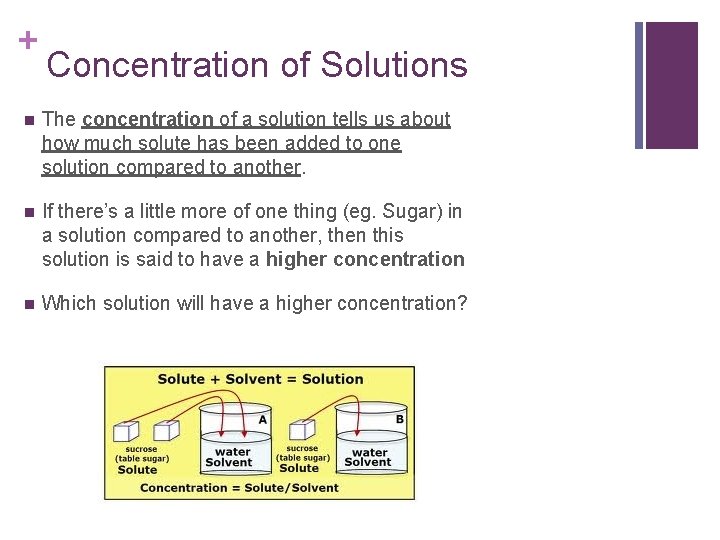
+ Concentration of Solutions n The concentration of a solution tells us about how much solute has been added to one solution compared to another. n If there’s a little more of one thing (eg. Sugar) in a solution compared to another, then this solution is said to have a higher concentration n Which solution will have a higher concentration?

+ Simple solutions n A simple solution is basically two substances that are going to be combined. n A solute is the substance to be dissolved (sugar). n The solvent is the one doing the dissolving (water). n As a rule of thumb, there is usually more solvent than solute. n Be patient with the next sentence as we put it all together. The amount of solute that can be dissolved by the solvent is defined as solubility. That's a lot of "sol" words.

+ Can anything be in a Solution? n Pretty much. Solutions can be solids dissolved in liquids. When you work with chemistry or even cook in your kitchen, you will usually be dissolving solids in liquids. n Solutions could also be gasses in other gases, and liquids in liquids. n If you mix two elements up and they are in an even distribution, they are a solution. n When elements are combined, but in unequal amounts, this is called a compound. n It is rare to find people making solid-sold solutions, but this is possible! How do you think a solid-solid solution would be created? n Solid-Solid solutions is usually a type of liquid/gas solution that has hardened!

+ SOLUTION EXAMPLE Gas-Gas Air Gas-Liquid Carbon Dixoide in Soda Liquid-Liquid Gasoline