Making Molar Solutions From Solids What are molar
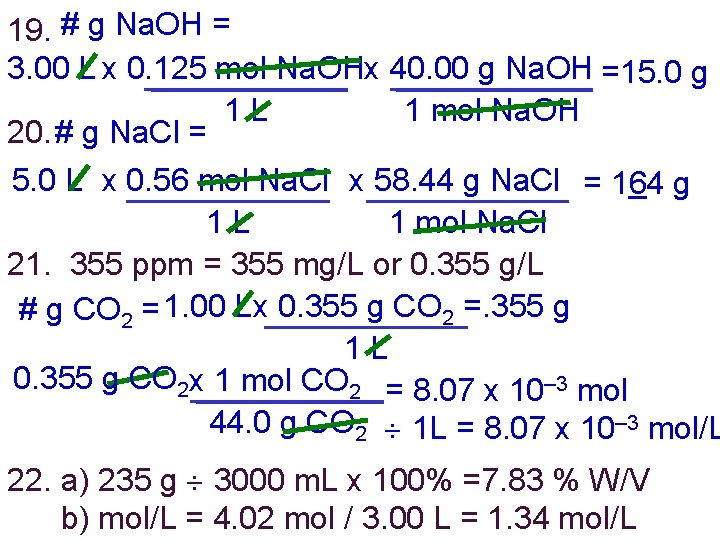


- Slides: 6

Making Molar Solutions From Solids

What are molar solutions? A molar solution is one that expresses “concentration” in moles per volume Usually the units are in mol/L can be abbreviated as M or [ ] Molar solutions are prepared using: a balance to weigh moles (as grams) a volumetric flask to measure litres L refers to entire volume, not water! Because the units are mol/L, we can use the equation M = n/L Alternatively, we can use the factor label method

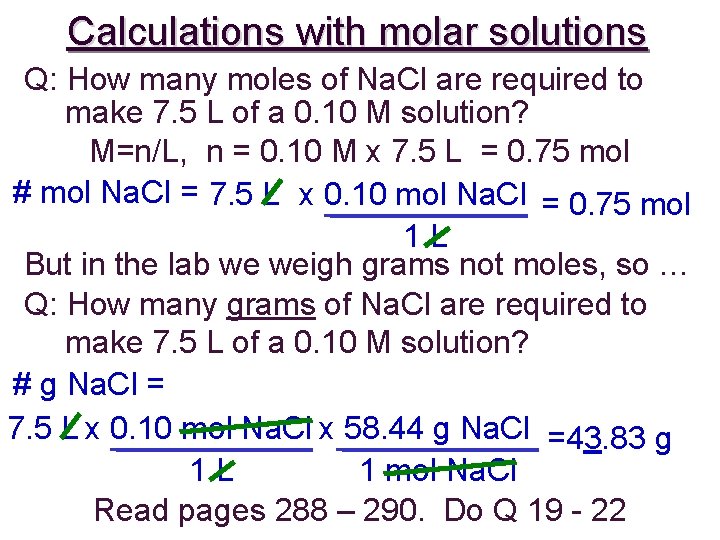
Calculations with molar solutions Q: How many moles of Na. Cl are required to make 7. 5 L of a 0. 10 M solution? M=n/L, n = 0. 10 M x 7. 5 L = 0. 75 mol # mol Na. Cl = 7. 5 L x 0. 10 mol Na. Cl = 0. 75 mol 1 L But in the lab we weigh grams not moles, so … Q: How many grams of Na. Cl are required to make 7. 5 L of a 0. 10 M solution? # g Na. Cl = 7. 5 L x 0. 10 mol Na. Cl x 58. 44 g Na. Cl =43. 83 g 1 L 1 mol Na. Cl Read pages 288 – 290. Do Q 19 - 22
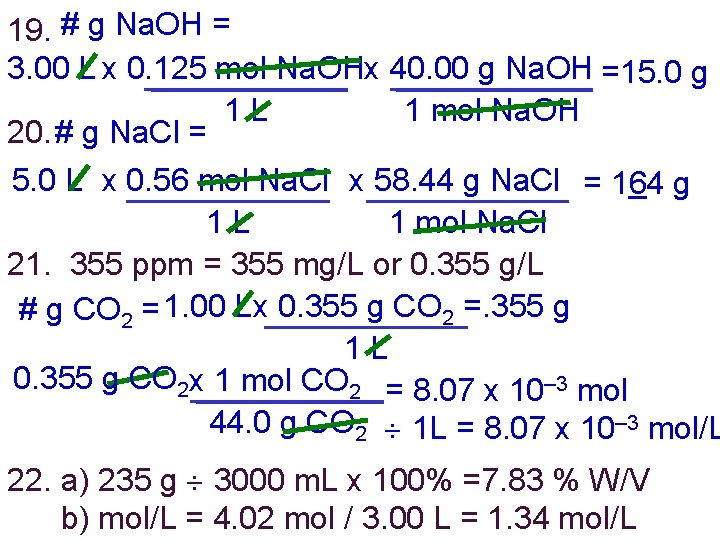
19. # g Na. OH = 3. 00 L x 0. 125 mol Na. OHx 40. 00 g Na. OH =15. 0 g 1 L 1 mol Na. OH 20. # g Na. Cl = 5. 0 L x 0. 56 mol Na. Cl x 58. 44 g Na. Cl = 164 g 1 L 1 mol Na. Cl 21. 355 ppm = 355 mg/L or 0. 355 g/L # g CO 2 = 1. 00 Lx 0. 355 g CO 2 =. 355 g 1 L 0. 355 g CO 2 x 1 mol CO 2 = 8. 07 x 10– 3 mol 44. 0 g CO 2 1 L = 8. 07 x 10– 3 mol/L 22. a) 235 g 3000 m. L x 100% =7. 83 % W/V b) mol/L = 4. 02 mol / 3. 00 L = 1. 34 mol/L

Practice making molar solutions 1. Calculate # of grams required to make 100 m. L of a 0. 10 M solution of Na. OH (see above). 2. Get volumetric flask, plastic bottle, 100 m. L beaker, eyedropper. Rinse all with tap water. 3. Fill a beaker with distilled water. 4. Pour 20 - 30 m. L of H 2 O from beaker into flask. 5. Weigh Na. OH. Add it to flask. Do step 5 quickly. 6. Mix (by swirling) until the Na. OH is dissolved. 7. Add distilled H 2 O to just below the colored line. 8. Add distilled H 2 O to the line using eyedropper. 9. Place solution in a bottle. Place label (tape) on bottle (name, date, chemical, molarity). Place bottle at front. Rinse & return equipment.

More Practice Questions For more lessons, visit www. chalkbored. com 1. How many grams of nitric acid are present in 1. 0 L of a 1. 0 M HNO 3 solution? 63 g 2. Calculate the number of grams needed to produce 1. 00 L of these solutions: a) 1. 00 M KNO 3 101 g b) 1. 85 M H 2 SO 4 181 g c) 0. 67 M KCl. O 3 82 g 3. Calculate the # of grams needed to produce each: a) 0. 20 L of 1. 5 M KCl b) 0. 160 L of 0. 300 M HCl c) 0. 20 L of 0. 09 mol/L Ag. NO 3 a) 22 g b) 1. 75 g d) 250 m. L of 3. 1 mol/L Ba. Cl 2 c) 3 g d) 0. 16 kg 4. Give the molarity of a solution containing 10 g of each solute in 2. 5 L of solution: a)H 2 SO 4 b)Ca(OH)2 5. Describe how 100 m. L of a 0. 10 mol/L a) 0. 041 mol/L Na. OH solution would be made. b) 0. 054 mol/L