Making Measurements Quantitative Observations The SI Metric System

Making Measurements (Quantitative Observations): The (SI) Metric System , Uncertainty, & Error
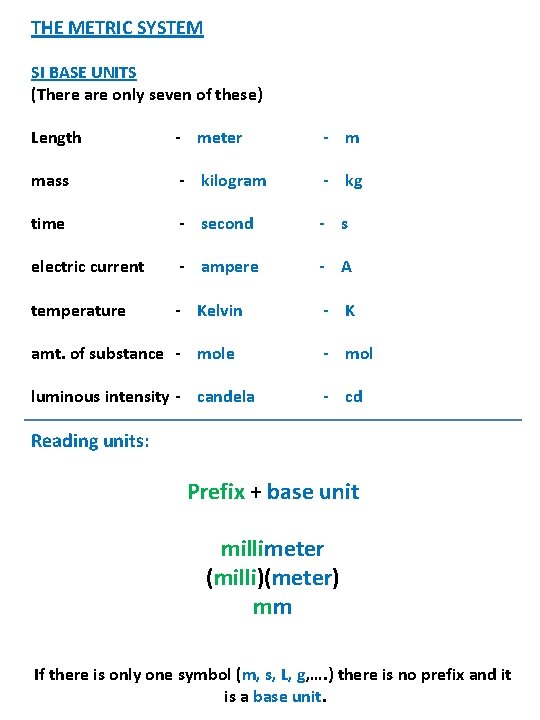
THE METRIC SYSTEM SI BASE UNITS (There are only seven of these) Length - meter - m mass - kilogram - kg time - second - s electric current - ampere - A temperature - Kelvin - K amt. of substance - mol luminous intensity - candela - cd Reading units: Prefix + base unit millimeter (milli)(meter) mm If there is only one symbol (m, s, L, g, …. ) there is no prefix and it is a base unit.
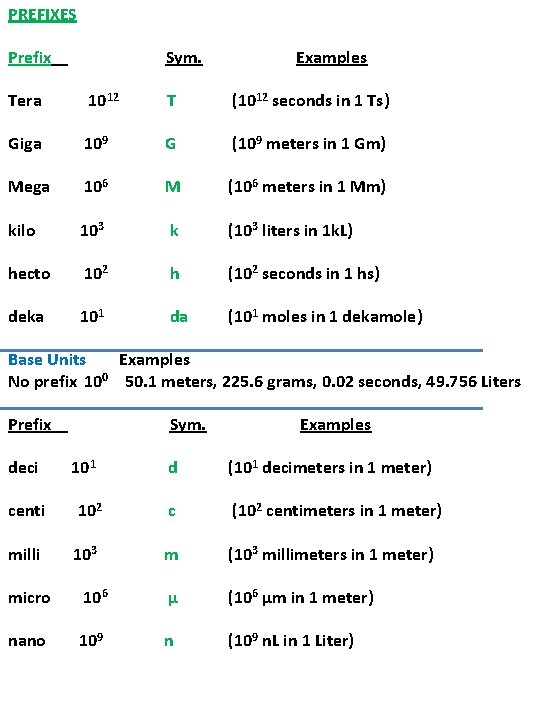
PREFIXES Prefix Sym. Examples Tera 1012 T (1012 seconds in 1 Ts) Giga 109 G (109 meters in 1 Gm) Mega 106 M (106 meters in 1 Mm) kilo 103 k (103 liters in 1 k. L) hecto 102 h (102 seconds in 1 hs) deka 101 da (101 moles in 1 dekamole) Base Units Examples No prefix 100 50. 1 meters, 225. 6 grams, 0. 02 seconds, 49. 756 Liters Prefix deci Sym. 101 Examples d (101 decimeters in 1 meter) centi 102 c (102 centimeters in 1 meter) milli 103 m (103 millimeters in 1 meter) micro 106 µ (106 µm in 1 meter) nano 109 n (109 n. L in 1 Liter)
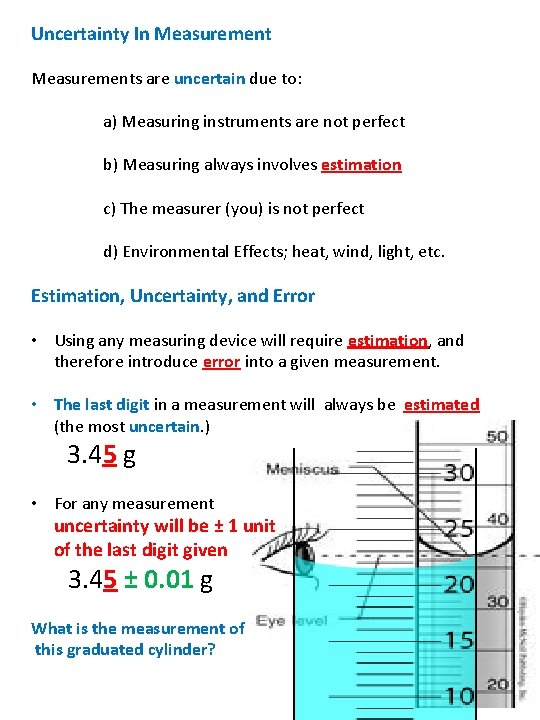
Uncertainty In Measurements are uncertain due to: a) Measuring instruments are not perfect b) Measuring always involves estimation c) The measurer (you) is not perfect d) Environmental Effects; heat, wind, light, etc. Estimation, Uncertainty, and Error • Using any measuring device will require estimation, and therefore introduce error into a given measurement. • The last digit in a measurement will always be estimated (the most uncertain. ) 3. 45 g • For any measurement uncertainty will be ± 1 unit of the last digit given 3. 45 ± 0. 01 g What is the measurement of this graduated cylinder?
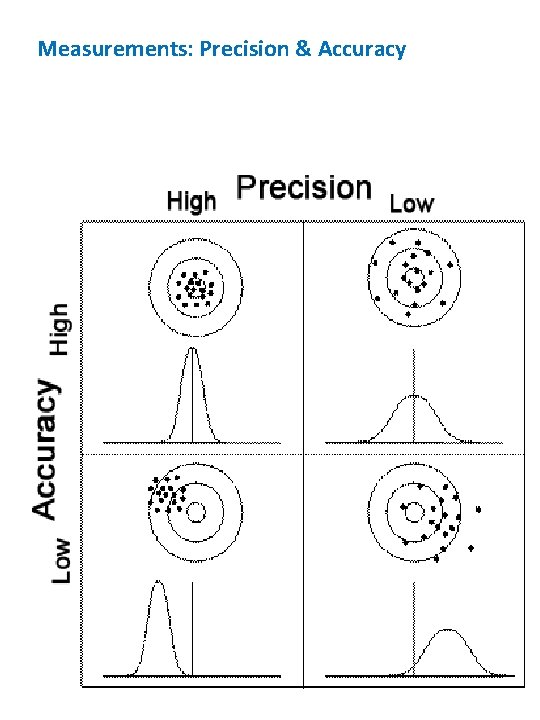
Measurements: Precision & Accuracy
- Slides: 5